Ionic Charge In Chlorine . Find out how to name and write ions using systematic and symbolic methods, and. Uncombined elements have an oxidation state of 0. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. See examples of sodium and chlorine ions, and. Find a comprehensive list of. See the periodic table with charges, element. It is defined as being the charge that an atom would have if all bonds were ionic. Learn how atoms lose or gain electrons to form ions with positive or negative charges. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn how atoms lose or gain electrons to become ions with different charges. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Find out how to use the atomic number, the valence shell and the periodic table to determine the.
from www.askiitians.com
93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Learn how atoms lose or gain electrons to become ions with different charges. Learn how atoms lose or gain electrons to form ions with positive or negative charges. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. See examples of sodium and chlorine ions, and. It is defined as being the charge that an atom would have if all bonds were ionic. Find out how to name and write ions using systematic and symbolic methods, and. See the periodic table with charges, element. Uncombined elements have an oxidation state of 0.
Chlorine Study Material for IIT JEE askIITians
Ionic Charge In Chlorine It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Uncombined elements have an oxidation state of 0. Find a comprehensive list of. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See the periodic table with charges, element. See examples of sodium and chlorine ions, and. Learn how atoms lose or gain electrons to form ions with positive or negative charges. It is defined as being the charge that an atom would have if all bonds were ionic. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Find out how to name and write ions using systematic and symbolic methods, and. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Find out how to use the atomic number, the valence shell and the periodic table to determine the. Learn how atoms lose or gain electrons to become ions with different charges.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Ionic Charge In Chlorine Find a comprehensive list of. Uncombined elements have an oxidation state of 0. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Find out how to use the atomic number, the valence. Ionic Charge In Chlorine.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Ionic Charge In Chlorine Find out how to name and write ions using systematic and symbolic methods, and. Find a comprehensive list of. See examples of sodium and chlorine ions, and. See the periodic table with charges, element. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Learn how atoms form cations and anions by losing. Ionic Charge In Chlorine.
From slideplayer.com
What are ionic compounds and how do they form? ppt download Ionic Charge In Chlorine 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. It is defined as being the charge that an atom would have if all bonds were ionic. Learn how atoms lose or gain electrons to form ions with positive or negative charges. See the periodic table with charges, element.. Ionic Charge In Chlorine.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Ionic Charge In Chlorine Learn how atoms lose or gain electrons to form ions with positive or negative charges. See the periodic table with charges, element. Uncombined elements have an oxidation state of 0. Find out how to use the atomic number, the valence shell and the periodic table to determine the. It is defined as being the charge that an atom would have. Ionic Charge In Chlorine.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Ionic Charge In Chlorine See the periodic table with charges, element. See examples of sodium and chlorine ions, and. It is defined as being the charge that an atom would have if all bonds were ionic. Find out how to name and write ions using systematic and symbolic methods, and. Learn how atoms lose or gain electrons to become ions with different charges. Find. Ionic Charge In Chlorine.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Ionic Charge In Chlorine Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. See the periodic table with charges, element. Find a comprehensive list of. Find out how to use the atomic number, the valence shell and the periodic table to determine the. Learn how atoms form cations and anions by losing or. Ionic Charge In Chlorine.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision Ionic Charge In Chlorine See the periodic table with charges, element. See examples of sodium and chlorine ions, and. Find out how to name and write ions using systematic and symbolic methods, and. Find a comprehensive list of. Learn how atoms lose or gain electrons to form ions with positive or negative charges. It is defined as being the charge that an atom would. Ionic Charge In Chlorine.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Ionic Charge In Chlorine Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See examples of sodium and chlorine ions, and. Find out how to name and write ions using systematic and symbolic methods, and. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. It is defined as being. Ionic Charge In Chlorine.
From en.wikipedia.org
Ionic bonding Wikipedia Ionic Charge In Chlorine 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Find a comprehensive. Ionic Charge In Chlorine.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Ionic Charge In Chlorine It is defined as being the charge that an atom would have if all bonds were ionic. See examples of sodium and chlorine ions, and. Uncombined elements have an oxidation state of 0. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. 93 rows find the most common charges for atoms of. Ionic Charge In Chlorine.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Ionic Charge In Chlorine Find out how to use the atomic number, the valence shell and the periodic table to determine the. Learn how atoms lose or gain electrons to form ions with positive or negative charges. Find out how to name and write ions using systematic and symbolic methods, and. Learn how atoms lose or gain electrons to become ions with different charges.. Ionic Charge In Chlorine.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Ionic Charge In Chlorine Uncombined elements have an oxidation state of 0. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. See the periodic table with charges, element. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows learn how to calculate ionic charges of elements based on. Ionic Charge In Chlorine.
From www.expii.com
Ions — Definition & Overview Expii Ionic Charge In Chlorine It is defined as being the charge that an atom would have if all bonds were ionic. See the periodic table with charges, element. Learn how atoms lose or gain electrons to become ions with different charges. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Find out how. Ionic Charge In Chlorine.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Ionic Charge In Chlorine See examples of sodium and chlorine ions, and. Learn how atoms lose or gain electrons to form ions with positive or negative charges. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. 93 rows learn how to calculate ionic charges of elements based on their electron gain or. Ionic Charge In Chlorine.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Ionic Charge In Chlorine Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Find out how to name and write ions using systematic and symbolic methods, and. Learn how atoms lose or gain electrons to become. Ionic Charge In Chlorine.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Ionic Charge In Chlorine Find a comprehensive list of. Uncombined elements have an oxidation state of 0. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. It is defined as being the charge that an atom would have if all bonds were ionic. See examples of sodium and chlorine ions, and. Find out how to name. Ionic Charge In Chlorine.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4192871 Ionic Charge In Chlorine It is defined as being the charge that an atom would have if all bonds were ionic. Find out how to name and write ions using systematic and symbolic methods, and. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. 93 rows find the most common charges for atoms of the chemical. Ionic Charge In Chlorine.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Ionic Charge In Chlorine 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Find out how to name and write ions using systematic and symbolic methods, and. Learn how atoms form cations. Ionic Charge In Chlorine.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Ionic Charge In Chlorine 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. See the periodic table with charges, element. Learn how atoms lose or gain electrons to form ions with positive or negative charges. Learn how ions form when electrons move from one atom to another, and how to name and. Ionic Charge In Chlorine.
From giogcjbix.blob.core.windows.net
Configuration Of Chlorine Valence Electrons at David Carver blog Ionic Charge In Chlorine Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Learn how. Ionic Charge In Chlorine.
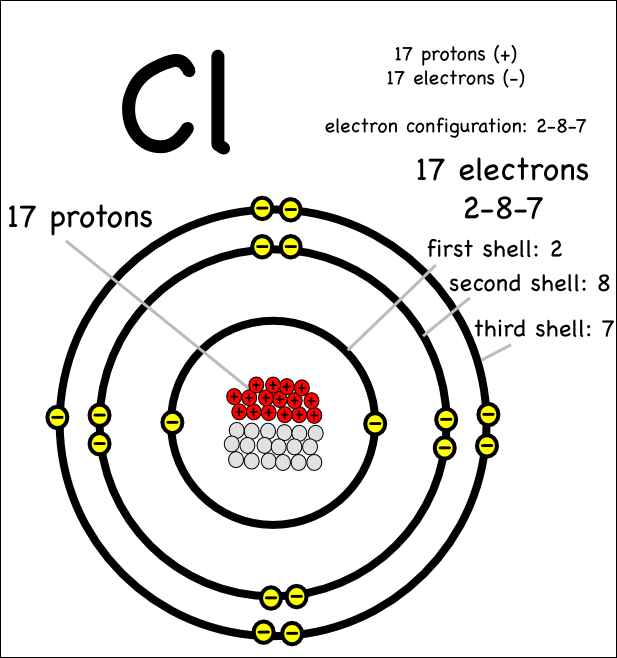
From loeylrncp.blob.core.windows.net
Chlorine Electrons And Protons at Edward Thompson blog Ionic Charge In Chlorine Find a comprehensive list of. Learn how atoms lose or gain electrons to form ions with positive or negative charges. See the periodic table with charges, element. Learn how atoms lose or gain electrons to become ions with different charges. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas.. Ionic Charge In Chlorine.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Ionic Charge In Chlorine Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows learn how to calculate. Ionic Charge In Chlorine.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Ionic Charge In Chlorine See examples of sodium and chlorine ions, and. Find out how to name and write ions using systematic and symbolic methods, and. It is defined as being the charge that an atom would have if all bonds were ionic. Uncombined elements have an oxidation state of 0. See the periodic table with charges, element. Learn how ions form when electrons. Ionic Charge In Chlorine.
From littleeagles.edu.vn
How To Find The Protons, Neutrons And Electrons For Chlorine? Ionic Charge In Chlorine Uncombined elements have an oxidation state of 0. 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Learn how atoms lose or gain electrons to become ions with different charges. See the periodic table with charges, element. It is defined as being the charge that an atom would have if all bonds. Ionic Charge In Chlorine.
From www2.victoriacollege.edu
formation of ionic bonds Ionic Charge In Chlorine Learn how atoms lose or gain electrons to form ions with positive or negative charges. Learn how atoms lose or gain electrons to become ions with different charges. Find a comprehensive list of. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Find out how to use the. Ionic Charge In Chlorine.
From slideplayer.com
Chemical Formulas and Chemical Bonding ppt download Ionic Charge In Chlorine 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Find a comprehensive list of. Find out how to name and write ions using systematic and symbolic methods, and. Learn how atoms lose or gain electrons to form ions with positive or negative charges. See the periodic table with charges, element. Learn how. Ionic Charge In Chlorine.
From saylordotorg.github.io
Ionic Bonding and Simple Ionic Compounds Ionic Charge In Chlorine 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. See the periodic table with charges, element. Find a comprehensive list of. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. See examples of sodium and chlorine ions,. Ionic Charge In Chlorine.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Ionic Charge In Chlorine Uncombined elements have an oxidation state of 0. Learn how atoms lose or gain electrons to become ions with different charges. See examples of sodium and chlorine ions, and. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. It is defined as being the charge that an atom would. Ionic Charge In Chlorine.
From slideplayer.com
Stable Electron Configurations ppt download Ionic Charge In Chlorine It is defined as being the charge that an atom would have if all bonds were ionic. Learn how atoms lose or gain electrons to become ions with different charges. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Learn how atoms form cations and anions by losing or. Ionic Charge In Chlorine.
From slideplayer.com
Chemical Formulas and Chemical Bonding ppt download Ionic Charge In Chlorine See the periodic table with charges, element. Find out how to name and write ions using systematic and symbolic methods, and. It is defined as being the charge that an atom would have if all bonds were ionic. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Uncombined elements have an oxidation state. Ionic Charge In Chlorine.
From wirelistcollegium.z14.web.core.windows.net
Lewis Dot Diagram For Ionic Bonds Ionic Charge In Chlorine 93 rows learn how to calculate ionic charges of elements based on their electron gain or loss. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. Learn how ions form when electrons. Ionic Charge In Chlorine.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Ionic Charge In Chlorine Find a comprehensive list of. Uncombined elements have an oxidation state of 0. See examples of sodium and chlorine ions, and. See the periodic table with charges, element. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Find out how to name and write ions using systematic and symbolic methods, and. Learn how. Ionic Charge In Chlorine.
From gioamsorw.blob.core.windows.net
Chlorine Ion Formed Name at Thomas Horton blog Ionic Charge In Chlorine Learn how atoms lose or gain electrons to form ions with positive or negative charges. It is defined as being the charge that an atom would have if all bonds were ionic. 93 rows find the most common charges for atoms of the chemical elements based on their valence electrons or oxidation states. See examples of sodium and chlorine ions,. Ionic Charge In Chlorine.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Ionic Charge In Chlorine Learn how atoms lose or gain electrons to form ions with positive or negative charges. Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. Find out how to use the atomic number, the valence shell and the periodic table to determine the. Learn how atoms lose or gain electrons. Ionic Charge In Chlorine.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Ionic Charge In Chlorine Learn how ions form when electrons move from one atom to another, and how to name and write ionic formulas. See examples of sodium and chlorine ions, and. Learn how atoms form cations and anions by losing or gaining electrons to achieve an octet. Find out how to name and write ions using systematic and symbolic methods, and. Uncombined elements. Ionic Charge In Chlorine.