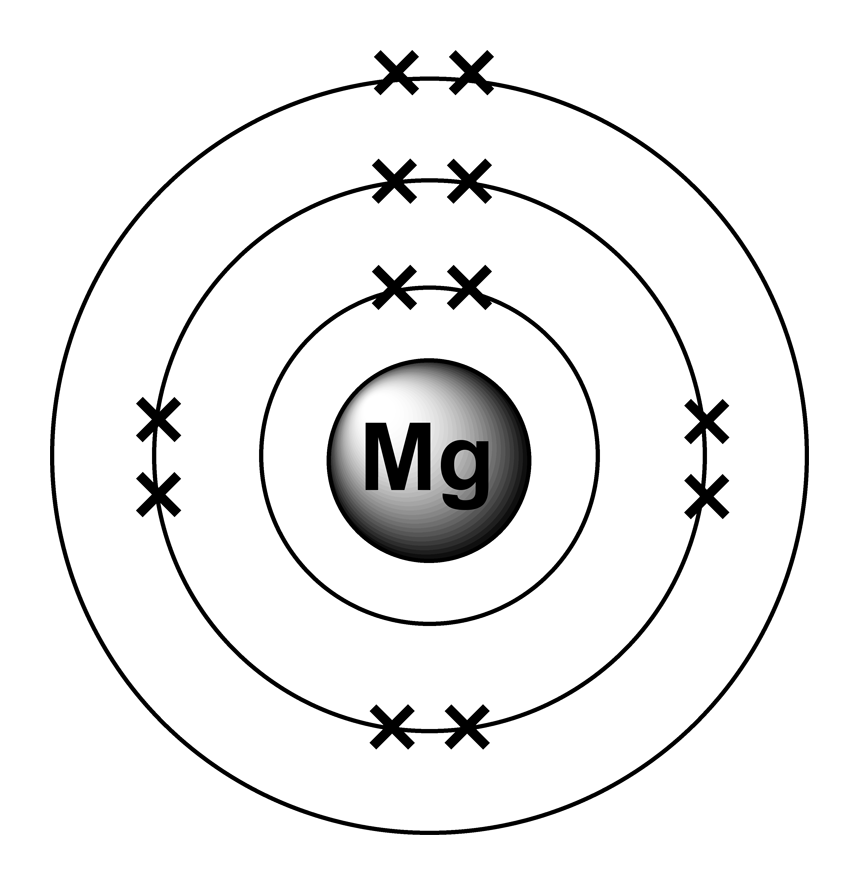
What Is Magnesium Bohr Model . magnesium has 12 protons and 12 electrons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. In the nucleus you would. magnesium has 3 energy levels: the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. The following are his key contributions to. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg).
from gionbptem.blob.core.windows.net
the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. The first electron shell of a bohr model holds 2 electrons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 12 protons and 12 electrons. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. magnesium has 3 energy levels: in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. In the nucleus you would.
Bohr Model Of Magnesium Ion at Stacey Allen blog
What Is Magnesium Bohr Model magnesium has 3 energy levels: magnesium has 12 protons and 12 electrons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The following are his key contributions to. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. magnesium has 3 energy levels: In the nucleus you would. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can.
From gionbptem.blob.core.windows.net
Bohr Model Of Magnesium Ion at Stacey Allen blog What Is Magnesium Bohr Model In the nucleus you would. magnesium has 12 protons and 12 electrons. The following are his key contributions to. The first electron shell of a bohr model holds 2 electrons. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in 1913, the danish physicist niels bohr proposed a model. What Is Magnesium Bohr Model.
From la.wikipedia.org
FasciculusElectron shell 012 magnesium.png Vicipaedia What Is Magnesium Bohr Model In the nucleus you would. The following are his key contributions to. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can.. What Is Magnesium Bohr Model.
From mavink.com
Magnesium Bohr Model Diagram What Is Magnesium Bohr Model 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. The following are his key contributions to. in this video we'll look at. What Is Magnesium Bohr Model.
From stock.adobe.com
Bohr model diagram of magnesium in atomic physics Stock Vector Adobe Stock What Is Magnesium Bohr Model the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. magnesium has 12 protons and 12 electrons. magnesium has 3 energy levels: bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. . What Is Magnesium Bohr Model.
From www.animalia-life.club
Bohr Model Labeled What Is Magnesium Bohr Model the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. bohr incorporated planck’s and einstein’s quantization ideas into a model of. What Is Magnesium Bohr Model.
From keywordsuggest.org
Image Gallery magnesium model What Is Magnesium Bohr Model magnesium has 3 energy levels: in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. magnesium has 12 protons and 12 electrons. 2 electrons in the 1st level, 8 electrons. What Is Magnesium Bohr Model.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube What Is Magnesium Bohr Model in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). In the nucleus you would. magnesium has 3 energy levels: bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. bohr’s model of the hydrogen. What Is Magnesium Bohr Model.
From ar.inspiredpencil.com
Magnesium Ion Bohr Model What Is Magnesium Bohr Model The following are his key contributions to. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. magnesium has 3 energy levels: . What Is Magnesium Bohr Model.
From exyozlpcg.blob.core.windows.net
Bohr Model Diagram Of Magnesium at Victoria Cummings blog What Is Magnesium Bohr Model in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). The first electron shell of a bohr model holds 2 electrons. magnesium has 12 protons and 12 electrons. In the nucleus you would. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level.. What Is Magnesium Bohr Model.
From enginelistjuprefecture.z21.web.core.windows.net
Magnesium Atom Diagram What Is Magnesium Bohr Model The following are his key contributions to. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. In the nucleus you would. bohr incorporated. What Is Magnesium Bohr Model.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and molecules for kids What Is Magnesium Bohr Model in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 3 energy levels: The first electron shell of a bohr model. What Is Magnesium Bohr Model.
From ar.inspiredpencil.com
Bohr Model Of Magnesium What Is Magnesium Bohr Model the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission. What Is Magnesium Bohr Model.
From www.dreamstime.com
Magnesium atom Bohr model stock vector. Illustration of background 267662111 What Is Magnesium Bohr Model bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete. What Is Magnesium Bohr Model.
From www.slideserve.com
PPT Bohr models PowerPoint Presentation, free download ID3062580 What Is Magnesium Bohr Model In the nucleus you would. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. bohr’s. What Is Magnesium Bohr Model.
From www.shutterstock.com
Bohr Model Magnesium Atom Electron Structure Stock Vector (Royalty Free) 1933399232 Shutterstock What Is Magnesium Bohr Model in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. The following are his key contributions to. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. In the nucleus you would.. What Is Magnesium Bohr Model.
From www.sliderbase.com
Bohr Models and Lewis Dot Diagrams Presentation Chemistry What Is Magnesium Bohr Model 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able to. magnesium has 3 energy levels: magnesium has 12 protons and 12 electrons.. What Is Magnesium Bohr Model.
From brainly.com
This is Bohr's Model of A. Oxygen B. Magnesium C. Sulfur What Is Magnesium Bohr Model The first electron shell of a bohr model holds 2 electrons. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). bohr incorporated planck’s and einstein’s quantization ideas into a model. What Is Magnesium Bohr Model.
From mungfali.com
Magnesium Bohr Model What Is Magnesium Bohr Model The first electron shell of a bohr model holds 2 electrons. magnesium has 12 protons and 12 electrons. The following are his key contributions to. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg).. What Is Magnesium Bohr Model.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium My XXX Hot Girl What Is Magnesium Bohr Model magnesium has 3 energy levels: The first electron shell of a bohr model holds 2 electrons. The following are his key contributions to. In the nucleus you would. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. in this video we'll look at the atomic structure and bohr model for the. What Is Magnesium Bohr Model.
From www.dreamstime.com
Bohr Model of the Magnesium Atom Stock Illustration Illustration of proton, magnesium 283185986 What Is Magnesium Bohr Model magnesium has 3 energy levels: The first electron shell of a bohr model holds 2 electrons. In the nucleus you would. The following are his key contributions to. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. the bohr model, introduced by. What Is Magnesium Bohr Model.
From ar.inspiredpencil.com
Bohr Model Of Magnesium What Is Magnesium Bohr Model The following are his key contributions to. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The first electron shell of a bohr model holds 2 electrons. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus. What Is Magnesium Bohr Model.
From mungfali.com
Magnesium Bohr Model What Is Magnesium Bohr Model magnesium has 3 energy levels: bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The following are his key contributions to. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in 1913, the danish physicist niels bohr proposed a model of the. What Is Magnesium Bohr Model.
From www.shutterstock.com
Bohr Model Representation Magnesium Atom Number Stock Vector (Royalty Free) 1999370114 What Is Magnesium Bohr Model bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. In the nucleus you would. The following are his key contributions to. in 1913,. What Is Magnesium Bohr Model.
From www.americanelements.com
Magnesium (Mg) AMERICAN ELEMENTSs What Is Magnesium Bohr Model magnesium has 12 protons and 12 electrons. In the nucleus you would. The following are his key contributions to. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The first electron shell of a bohr model holds 2 electrons. magnesium has 3 energy levels: in this video we'll look at. What Is Magnesium Bohr Model.
From www.slideserve.com
PPT Bohr Models PowerPoint Presentation, free download ID949135 What Is Magnesium Bohr Model magnesium has 12 protons and 12 electrons. magnesium has 3 energy levels: 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The following are his key contributions to. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. . What Is Magnesium Bohr Model.
From mungfali.com
Magnesium Atom Bohr Model What Is Magnesium Bohr Model in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability. What Is Magnesium Bohr Model.
From gionbptem.blob.core.windows.net
Bohr Model Of Magnesium Ion at Stacey Allen blog What Is Magnesium Bohr Model bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus. What Is Magnesium Bohr Model.
From fyoxsjjaa.blob.core.windows.net
Magnesium Bohr at William Bigger blog What Is Magnesium Bohr Model bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. The first electron shell of a bohr model holds 2 electrons. bohr incorporated planck’s and einstein’s quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. in this video we'll look at the. What Is Magnesium Bohr Model.
From www.shutterstock.com
Diagrama modelo Bohr de magnesio en vector de stock (libre de regalías) 2081491174 Shutterstock What Is Magnesium Bohr Model The following are his key contributions to. In the nucleus you would. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. The first electron shell of a bohr model holds 2. What Is Magnesium Bohr Model.
From www.showme.com
Bohr Model for Magnesium Science, Chemistry, Atoms, Elements ShowMe What Is Magnesium Bohr Model In the nucleus you would. magnesium has 3 energy levels: the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The following are his key contributions to. in this video. What Is Magnesium Bohr Model.
From mavink.com
Magnesium Bohr Model Diagram What Is Magnesium Bohr Model The first electron shell of a bohr model holds 2 electrons. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. in this video we'll look at the atomic structure and bohr model for the. What Is Magnesium Bohr Model.
From www.shutterstock.com
Bohr Model Magnesium Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 1933397057 What Is Magnesium Bohr Model 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. The following are his key contributions to. in this video we'll look at the atomic structure and bohr model for the magnesium atom (mg). magnesium has 12 protons and 12 electrons. the bohr model, introduced by niels bohr, describes. What Is Magnesium Bohr Model.
From mungfali.com
Magnesium Atom Bohr Model What Is Magnesium Bohr Model magnesium has 3 energy levels: bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom. What Is Magnesium Bohr Model.
From ar.inspiredpencil.com
Bohr Model For Magnesium What Is Magnesium Bohr Model magnesium has 12 protons and 12 electrons. the bohr model, introduced by niels bohr, describes the atom’s orbitals and the maximum number of electrons they can. In the nucleus you would. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in which electrons orbit the nucleus and were able. What Is Magnesium Bohr Model.
From giozhverl.blob.core.windows.net
Magnesium Long Electron Configuration at Michael Woods blog What Is Magnesium Bohr Model The following are his key contributions to. 2 electrons in the 1st level, 8 electrons in the 2nd and 2 electrons in the 3rd level. bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. in 1913, the danish physicist niels bohr proposed a model of the electron cloud of an atom in. What Is Magnesium Bohr Model.