Borax Titration Lab Report . Bromocresol green is blue in basic. Titrate the samples with the standardized hcl solution. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. Record the initial and final buret reading to ±0.01 ml. Calculate the ksp of each borax solution at the various temperatures. After adding approximately 25 ml of. Weigh accurately using an analytical balance and assess random errors. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Determination of thermodynamic parameters of borax. To determine h and s, make a plot of the experimental data on excel. On completion of this lab you should be able to: The relationship between ksp, gibb's free energy, enthalpy and entropy will be.
from www.studocu.com
Determination of thermodynamic parameters of borax. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Bromocresol green is blue in basic. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Record the initial and final buret reading to ±0.01 ml. Calculate the ksp of each borax solution at the various temperatures. After adding approximately 25 ml of. Titrate the samples with the standardized hcl solution. On completion of this lab you should be able to:
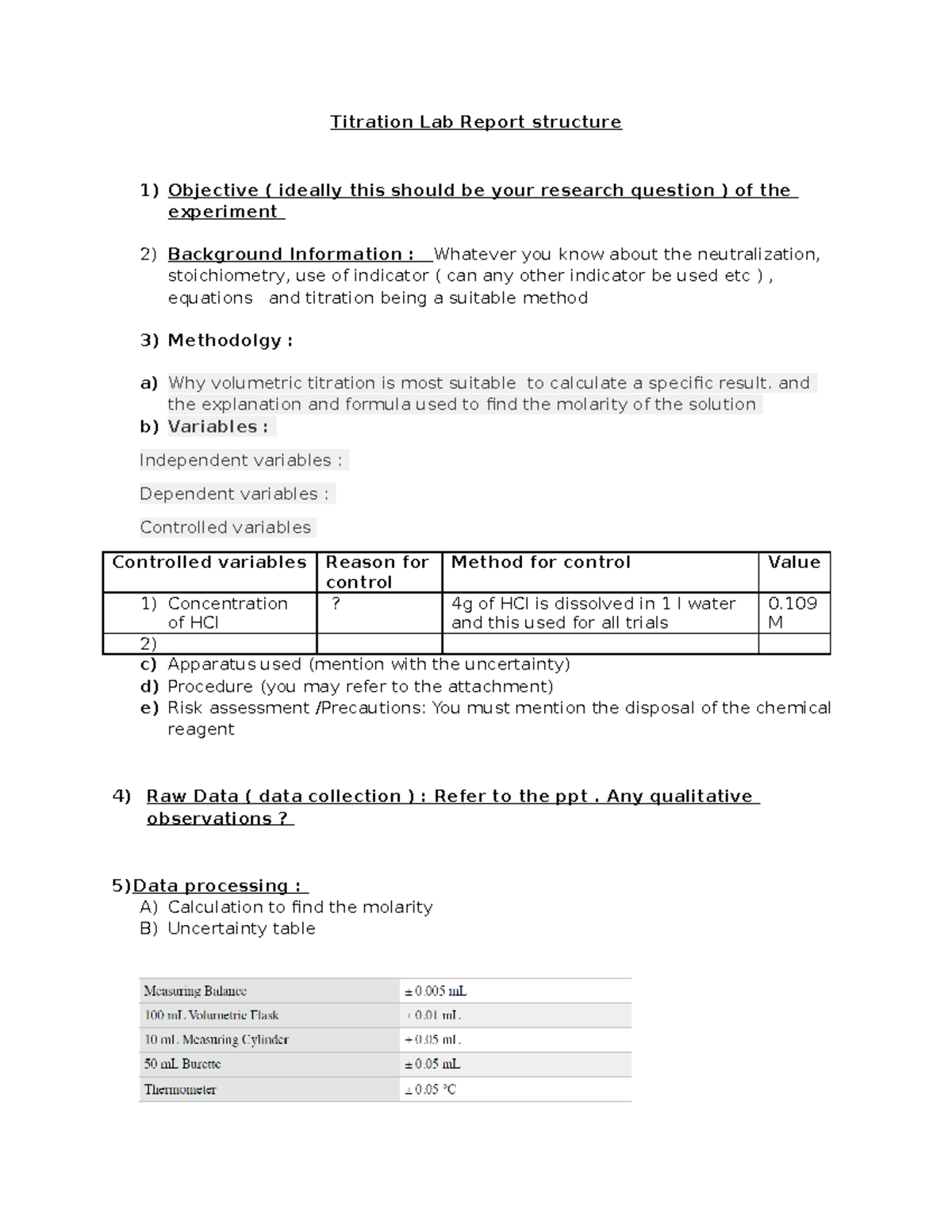
Titration Lab report format Titration Lab Report structure Objective
Borax Titration Lab Report To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Record the initial and final buret reading to ±0.01 ml. Determination of thermodynamic parameters of borax. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Bromocresol green is blue in basic. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. Weigh accurately using an analytical balance and assess random errors. On completion of this lab you should be able to: After adding approximately 25 ml of. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. To determine h and s, make a plot of the experimental data on excel. Titrate the samples with the standardized hcl solution. Calculate the ksp of each borax solution at the various temperatures.
From www.chegg.com
Solved Lab 19 Thermodynamics of the Dissolution of Borax Borax Titration Lab Report The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Calculate the ksp of each borax solution at the various temperatures. Bromocresol green is blue in basic. The objectives of this lab were to determine the values of enthalpy,. Borax Titration Lab Report.
From www.studocu.com
Borax experiment lab report Determining Thermodynamic Constants for Borax Titration Lab Report Weigh accurately using an analytical balance and assess random errors. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Following the procedure in this. Borax Titration Lab Report.
From www.chegg.com
Solved Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report After adding approximately 25 ml of. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Titrate the samples with the standardized hcl solution. To determine h and s, make a plot of the experimental data on excel. Bromocresol green is blue in basic. Record the initial and final buret reading to ±0.01 ml. The objectives of this. Borax Titration Lab Report.
From www.youtube.com
pH Titration Part 1/3 Preparing a borax standard YouTube Borax Titration Lab Report Determination of thermodynamic parameters of borax. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Record the initial and final buret reading to ±0.01 ml. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the. Borax Titration Lab Report.
From mungfali.com
Titration Lab Diagram Borax Titration Lab Report On completion of this lab you should be able to: Determination of thermodynamic parameters of borax. After adding approximately 25 ml of. Bromocresol green is blue in basic. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Record. Borax Titration Lab Report.
From www.chegg.com
Solved Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. To determine h and s, make a plot of the experimental data on excel. Calculate the ksp of each borax solution at the various temperatures. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy. Borax Titration Lab Report.
From www.scribd.com
Thermodynamics of The Dissolution of Borax Lab Report PDF Borax Titration Lab Report The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Calculate the ksp of each borax solution at the various temperatures. On completion of this lab you should be able to: To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Following the procedure in this week’s lab, 5.0 ml. Borax Titration Lab Report.
From www.chegg.com
Solved Mass of borax originally measured___ Concentration Borax Titration Lab Report The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. To determine h and s, make a plot of the experimental data on excel. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. After. Borax Titration Lab Report.
From www.docsity.com
Experiment Titration Lab Reports Chemistry Docsity Borax Titration Lab Report Bromocresol green is blue in basic. Weigh accurately using an analytical balance and assess random errors. On completion of this lab you should be able to: Titrate the samples with the standardized hcl solution. After adding approximately 25 ml of. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Following the. Borax Titration Lab Report.
From www.studocu.com
Borax Equilibrium Lab Report BIO 211 Studocu Borax Titration Lab Report Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. On completion of this lab you should be able to: The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine. Borax Titration Lab Report.
From mungfali.com
Titration Lab Diagram Borax Titration Lab Report Determination of thermodynamic parameters of borax. To determine h and s, make a plot of the experimental data on excel. Bromocresol green is blue in basic. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. Record. Borax Titration Lab Report.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Borax Titration Lab Report The relationship between ksp, gibb's free energy, enthalpy and entropy will be. On completion of this lab you should be able to: Titrate the samples with the standardized hcl solution. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. To determine the thermodynamic quantities δh° and δs° ,for the solvation. Borax Titration Lab Report.
From www.youtube.com
Thermodynamics of Borax Dissolution Intro & Theory YouTube Borax Titration Lab Report Record the initial and final buret reading to ±0.01 ml. Determination of thermodynamic parameters of borax. After adding approximately 25 ml of. To determine h and s, make a plot of the experimental data on excel. Weigh accurately using an analytical balance and assess random errors. The objectives of this lab were to determine the values of enthalpy, entropy and. Borax Titration Lab Report.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Borax Titration Lab Report Calculate the ksp of each borax solution at the various temperatures. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Titrate the samples with the standardized hcl solution. Record the initial and final buret reading to ±0.01 ml. The objectives of this lab were to determine the values of enthalpy, entropy. Borax Titration Lab Report.
From www.numerade.com
SOLVED Experiment 26 Report Shee of the Thermodynamics Borax Date Borax Titration Lab Report Determination of thermodynamic parameters of borax. To determine h and s, make a plot of the experimental data on excel. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Record the initial and final buret reading to ±0.01 ml. The relationship between ksp, gibb's free energy, enthalpy and entropy will. Borax Titration Lab Report.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Borax Titration Lab Report To determine h and s, make a plot of the experimental data on excel. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. On completion of this lab you should be able to: Bromocresol green is blue in basic. Record the initial and final buret reading to ±0.01 ml. Weigh. Borax Titration Lab Report.
From www.studocu.com
Thermodynamics of Dissolution of Borax lab report Name Thi Phuoc Borax Titration Lab Report Calculate the ksp of each borax solution at the various temperatures. Determination of thermodynamic parameters of borax. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Record the initial and final buret reading to ±0.01 ml. Bromocresol green is blue in basic. The objectives of this lab were to determine the. Borax Titration Lab Report.
From www.chegg.com
Solved Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report Record the initial and final buret reading to ±0.01 ml. Weigh accurately using an analytical balance and assess random errors. On completion of this lab you should be able to: Titrate the samples with the standardized hcl solution. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. To determine h. Borax Titration Lab Report.
From www.studocu.com
Borax Lab Report Sheet Borax Solubility Report Name Borax Titration Lab Report Determination of thermodynamic parameters of borax. Bromocresol green is blue in basic. Weigh accurately using an analytical balance and assess random errors. On completion of this lab you should be able to: Record the initial and final buret reading to ±0.01 ml. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43. Borax Titration Lab Report.
From www.numerade.com
SOLVED just need help with formulas. lost Lab Team Members TA Finding Borax Titration Lab Report The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. Weigh accurately using an analytical balance and assess random errors. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at. Borax Titration Lab Report.
From www.numerade.com
SOLVED Experimen Lah Tear Afcrbars Finding Equilibrium Constants The Borax Titration Lab Report Calculate the ksp of each borax solution at the various temperatures. Record the initial and final buret reading to ±0.01 ml. Determination of thermodynamic parameters of borax. To determine h and s, make a plot of the experimental data on excel. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Titrate the samples with the standardized hcl. Borax Titration Lab Report.
From www.chegg.com
Part A Titration of Room Temperature Borax KspI Borax Titration Lab Report To determine h and s, make a plot of the experimental data on excel. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to determine the equilibrium. Weigh accurately using an analytical balance and assess random errors. Record the initial. Borax Titration Lab Report.
From www.studocu.com
Lab 6. Determination of Thermodynamic parameters of Borax Dissolution Borax Titration Lab Report Determination of thermodynamic parameters of borax. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Titrate the samples with the standardized hcl solution. Record the initial and final buret reading to ±0.01 ml. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. After adding approximately 25 ml. Borax Titration Lab Report.
From www.studypool.com
SOLUTION Week base strong acid titration lab report and post lab Borax Titration Lab Report After adding approximately 25 ml of. Calculate the ksp of each borax solution at the various temperatures. To determine h and s, make a plot of the experimental data on excel. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy. Borax Titration Lab Report.
From www.studocu.com
Titration Lab report format Titration Lab Report structure Objective Borax Titration Lab Report Record the initial and final buret reading to ±0.01 ml. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. Calculate the ksp of each borax solution at the various temperatures. Bromocresol green is blue in basic. Weigh accurately using an analytical balance and assess random errors. Titrate the samples with the. Borax Titration Lab Report.
From www.chegg.com
Lab 9 Dissolution of Borax [Updated 03.03.2020] Data Borax Titration Lab Report To determine h and s, make a plot of the experimental data on excel. Titrate the samples with the standardized hcl solution. Record the initial and final buret reading to ±0.01 ml. Determination of thermodynamic parameters of borax. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax. Borax Titration Lab Report.
From www.studocu.com
Chem 2 Experiment 26 Thermodynamics of the Dissolution of Borax Table Borax Titration Lab Report Weigh accurately using an analytical balance and assess random errors. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. To determine the thermodynamic quantities δh° and δs° ,for the solvation reaction of borax in water by measuring. On completion of this lab you should be able to: Calculate the ksp of each borax solution at the various. Borax Titration Lab Report.
From www.chegg.com
Solved Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report Titrate the samples with the standardized hcl solution. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Weigh accurately using an analytical balance and assess random errors. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use these values to. Borax Titration Lab Report.
From www.chegg.com
Solved Lab 9 Dissolution of Borax [Updated 03.03.2020] Data Borax Titration Lab Report Record the initial and final buret reading to ±0.01 ml. Bromocresol green is blue in basic. After adding approximately 25 ml of. On completion of this lab you should be able to: Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. To determine h and s, make a plot of. Borax Titration Lab Report.
From www.chegg.com
Lab 9 Dissolution of Borax Data Analysis Table 2. Borax Titration Lab Report Record the initial and final buret reading to ±0.01 ml. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. After adding approximately 25 ml of. On completion of this lab you should be able to: The objectives of this lab were to determine the values of enthalpy, entropy and gibbs. Borax Titration Lab Report.
From www.scribd.com
titration lab report Titration Ph Borax Titration Lab Report Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Titrate the samples with the standardized hcl solution. Determination of thermodynamic parameters of borax. Calculate the ksp of each borax solution at the various temperatures. To determine h and s, make a plot of the experimental data on excel. Record the. Borax Titration Lab Report.
From www.chegg.com
Solved Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. To determine h and s, make a plot of the experimental data on excel. The objectives of this lab were to determine the values of enthalpy, entropy and gibbs free energy for the dissolution of borax in water, and then use. Borax Titration Lab Report.
From www.youtube.com
Analytical chemistry Determination of Boric Acid(H3BO3,Borax,Kjeldahl Borax Titration Lab Report Bromocresol green is blue in basic. Determination of thermodynamic parameters of borax. To determine h and s, make a plot of the experimental data on excel. On completion of this lab you should be able to: Weigh accurately using an analytical balance and assess random errors. Titrate the samples with the standardized hcl solution. Record the initial and final buret. Borax Titration Lab Report.
From www.chegg.com
Data Table 1 Titration of Saturated Borax Solution Borax Titration Lab Report To determine h and s, make a plot of the experimental data on excel. Determination of thermodynamic parameters of borax. Following the procedure in this week’s lab, 5.0 ml of a saturated borax solution was collected at 43 °c. Record the initial and final buret reading to ±0.01 ml. Bromocresol green is blue in basic. After adding approximately 25 ml. Borax Titration Lab Report.
From www.studocu.com
Final Borax LAB Report CHM 112 Studocu Borax Titration Lab Report Titrate the samples with the standardized hcl solution. The relationship between ksp, gibb's free energy, enthalpy and entropy will be. Determination of thermodynamic parameters of borax. Calculate the ksp of each borax solution at the various temperatures. Weigh accurately using an analytical balance and assess random errors. Following the procedure in this week’s lab, 5.0 ml of a saturated borax. Borax Titration Lab Report.