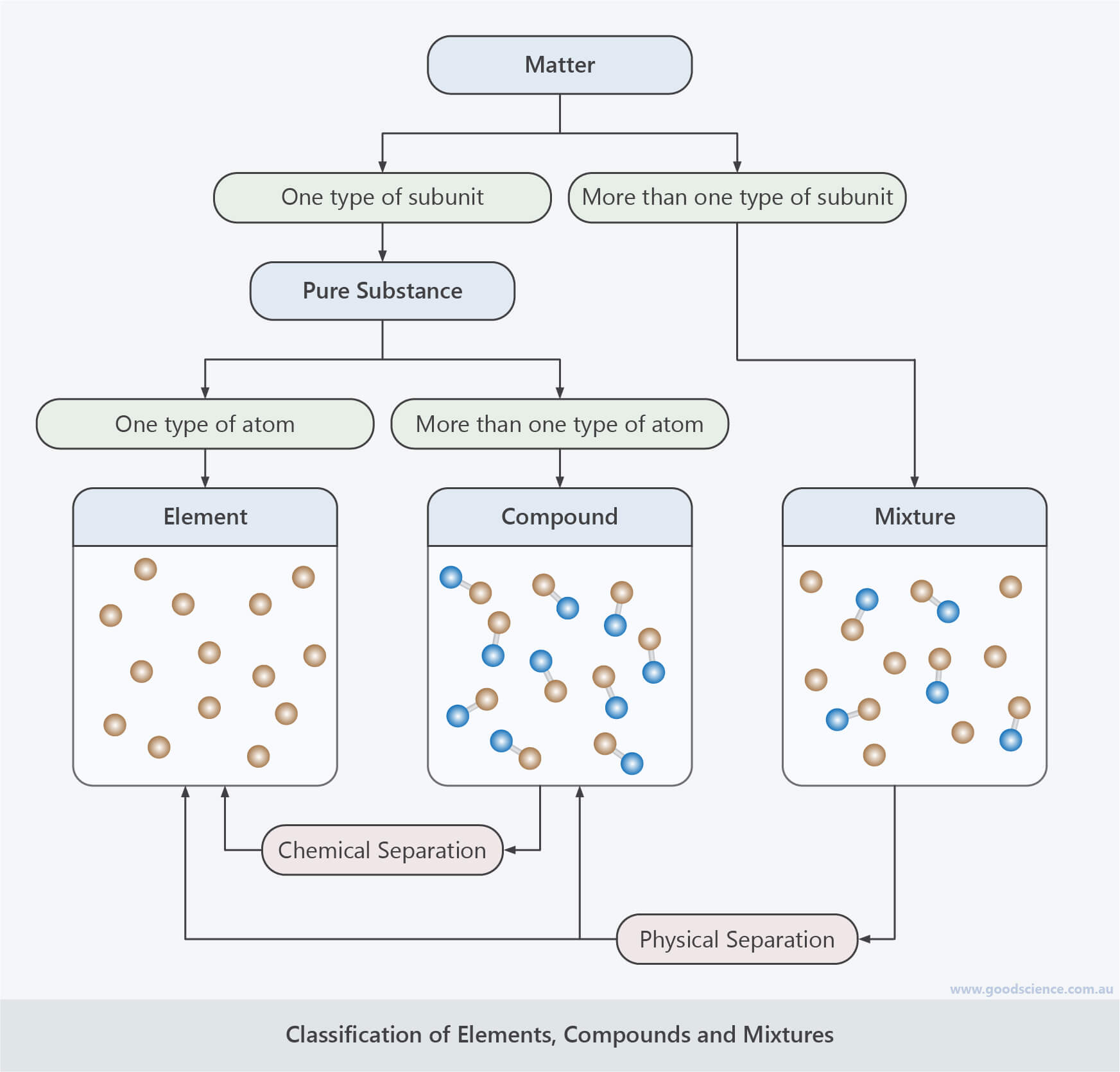
Is Pure Ice A Compound Element Or Mixture . If we take two or more pure substances and mix them together, we refer to this as a mixture. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Elements and compounds are both examples of pure substances. Ice is a compound, not a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. A mixture is a material which has two or more types of molecules. This is now a mixture of the elements iron and sulfur. However, impure water creates ice that’s considered a. When we mix two different pure substances together, like this, it’s a mixture. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. A compound is a pure substance which contains identical molecules with two or more types of atomic core. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Mixtures can be classified as homogeneous or heterogeneous. The different elements are not joined together.
from www.goodscience.com.au
Ice is a compound, not a mixture. Mixtures can be classified as homogeneous or heterogeneous. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. However, impure water creates ice that’s considered a. If we take two or more pure substances and mix them together, we refer to this as a mixture. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. A mixture is a material which has two or more types of molecules. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds.
Elements, Compounds and Mixtures Good Science
Is Pure Ice A Compound Element Or Mixture Elements and compounds are both examples of pure substances. A compound is a pure substance which contains identical molecules with two or more types of atomic core. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Mixtures can be classified as homogeneous or heterogeneous. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. The different elements are not joined together. Ice is a compound, not a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. A mixture is a material which has two or more types of molecules. When we mix two different pure substances together, like this, it’s a mixture. Elements and compounds are both examples of pure substances. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. If we take two or more pure substances and mix them together, we refer to this as a mixture. This is now a mixture of the elements iron and sulfur. However, impure water creates ice that’s considered a.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Is Pure Ice A Compound Element Or Mixture Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. A compound is a pure substance which contains identical molecules with two or more types of atomic core. This is now a mixture of the elements iron and sulfur. Elements and compounds are both examples of pure. Is Pure Ice A Compound Element Or Mixture.
From www.slideserve.com
PPT Classifying Matter Elements, Compounds, and Mixtures PowerPoint Is Pure Ice A Compound Element Or Mixture Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Ice is a compound, not a mixture. Elements and compounds are both examples of pure substances. However, impure water creates ice that’s considered a. If we take two or more pure substances and mix them together, we. Is Pure Ice A Compound Element Or Mixture.
From stock.adobe.com
illustration of chemistry, Pure Substances and Mixtures, element Is Pure Ice A Compound Element Or Mixture Elements and compounds are both examples of pure substances. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. A compound is a pure substance which contains identical molecules. Is Pure Ice A Compound Element Or Mixture.
From onesatu11.blogspot.com
How Is A Compound Different From A Mixture one satu Is Pure Ice A Compound Element Or Mixture Elements and compounds are both examples of pure substances. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. If we take two or more pure substances and mix them together, we refer to this as a mixture. The different elements are not joined together. Mixtures can. Is Pure Ice A Compound Element Or Mixture.
From circuitdiagramalexandra.z5.web.core.windows.net
Element Compound Mixture Diagram Is Pure Ice A Compound Element Or Mixture When we mix two different pure substances together, like this, it’s a mixture. If we take two or more pure substances and mix them together, we refer to this as a mixture. However, impure water creates ice that’s considered a. Ice is a compound, not a mixture. This is now a mixture of the elements iron and sulfur. For example,. Is Pure Ice A Compound Element Or Mixture.
From slidecourse.blogspot.com
Pure Substance Or Mixture Slide Course Is Pure Ice A Compound Element Or Mixture For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. A compound is a pure substance which contains identical molecules with two or more types of atomic core. This is now a mixture of the elements iron and sulfur. The different elements are not joined together. Ice is a compound,. Is Pure Ice A Compound Element Or Mixture.
From schematicdiagrampoukes.z13.web.core.windows.net
Diagram Of Mixture Is Pure Ice A Compound Element Or Mixture Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. Elements and compounds are both examples of pure substances. Looking at the left side of the chart, we can. Is Pure Ice A Compound Element Or Mixture.
From www.youtube.com
Pure Substances, Elements, Compounds, Homogenous & Heterogenous Mixture Is Pure Ice A Compound Element Or Mixture If we take two or more pure substances and mix them together, we refer to this as a mixture. When we mix two different pure substances together, like this, it’s a mixture. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. Mixtures can always be separated again into component pure substances, because bonding. Is Pure Ice A Compound Element Or Mixture.
From guidemanualephemerist.z14.web.core.windows.net
Diagram Of Elements Compounds And Mixtures Is Pure Ice A Compound Element Or Mixture Elements and compounds are both examples of pure substances. When we mix two different pure substances together, like this, it’s a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. A mixture is a material which has two or more types of molecules.. Is Pure Ice A Compound Element Or Mixture.
From mungfali.com
Element Compound And Mixture Chart Is Pure Ice A Compound Element Or Mixture For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Ice is a compound, not a mixture. When we mix two different pure substances together, like this,. Is Pure Ice A Compound Element Or Mixture.
From unacademy.com
Science Class 9 Pure Substance vs Mixture UPSC Note on Science Class Is Pure Ice A Compound Element Or Mixture When we mix two different pure substances together, like this, it’s a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. However, impure. Is Pure Ice A Compound Element Or Mixture.
From sciencing.com
How are Mixtures And Pure Substances Alike Sciencing Is Pure Ice A Compound Element Or Mixture Mixtures can be classified as homogeneous or heterogeneous. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. The different elements are not joined together. A compound is a pure substance which contains identical molecules with two or more types of atomic core. A mixture is a material which has. Is Pure Ice A Compound Element Or Mixture.
From mavink.com
Venn Diagram Elements Compounds And Mixtures Is Pure Ice A Compound Element Or Mixture If we take two or more pure substances and mix them together, we refer to this as a mixture. Mixtures can be classified as homogeneous or heterogeneous. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. A mixture is a material which has two. Is Pure Ice A Compound Element Or Mixture.
From stock.adobe.com
Stockillustratie classification of matter mixture (homogeneous and Is Pure Ice A Compound Element Or Mixture The different elements are not joined together. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Ice is water frozen into a solid state and comprises two elements —. Is Pure Ice A Compound Element Or Mixture.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Is Pure Ice A Compound Element Or Mixture Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. However, impure water creates ice that’s considered a. Ice is a compound, not a mixture. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Mixtures can be classified as. Is Pure Ice A Compound Element Or Mixture.
From temperaturemaster.com
Discovering the Chemical Composition of Ice Mixture or Compound? Is Pure Ice A Compound Element Or Mixture However, impure water creates ice that’s considered a. A mixture is a material which has two or more types of molecules. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. Elements and compounds are both examples of pure substances. Ice is a compound, not a mixture. Looking at the left side of the. Is Pure Ice A Compound Element Or Mixture.
From sciencetallis.weebly.com
8. Chemical Analysis THOMAS TALLIS SCIENCE Is Pure Ice A Compound Element Or Mixture The different elements are not joined together. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Mixtures can be classified as homogeneous or heterogeneous. However, impure water creates ice that’s considered a. Ice is a compound, not a mixture. Ice is water frozen into a solid state and comprises. Is Pure Ice A Compound Element Or Mixture.
From atscience.weebly.com
Elements, Compounds and Mixtures AT SCIENCE Is Pure Ice A Compound Element Or Mixture If we take two or more pure substances and mix them together, we refer to this as a mixture. The different elements are not joined together. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. When we mix two different pure substances together, like. Is Pure Ice A Compound Element Or Mixture.
From slidetodoc.com
Pure Substances and Mixtures Categorizing Matter All matter Is Pure Ice A Compound Element Or Mixture Mixtures can be classified as homogeneous or heterogeneous. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. A mixture is a material which has two or more types of molecules. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in. Is Pure Ice A Compound Element Or Mixture.
From www.yaclass.in
Differentiating pure substances and mixtures — lesson. Science State Is Pure Ice A Compound Element Or Mixture If we take two or more pure substances and mix them together, we refer to this as a mixture. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. A compound is a pure substance which contains identical molecules with two or more types of atomic core.. Is Pure Ice A Compound Element Or Mixture.
From www.teachoo.com
Pure Substances Meaning, Examples and Types Teachoo Concepts Is Pure Ice A Compound Element Or Mixture Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. When we mix two different pure substances together, like this, it’s a mixture. If we take two or more pure substances and mix them together, we refer to this as a mixture. A compound is a pure. Is Pure Ice A Compound Element Or Mixture.
From www.youtube.com
Pure Substances and Mixtures, Elements & Compounds, Classification of Is Pure Ice A Compound Element Or Mixture Elements and compounds are both examples of pure substances. The different elements are not joined together. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Looking at the left side of the chart, we can see that a pure substance can be either an. Is Pure Ice A Compound Element Or Mixture.
From temperaturemaster.com
What Category Does Dry Ice? Compound, Element, or Mixture Is Pure Ice A Compound Element Or Mixture When we mix two different pure substances together, like this, it’s a mixture. Mixtures can be classified as homogeneous or heterogeneous. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. A mixture is a material which has two or more types of molecules. However,. Is Pure Ice A Compound Element Or Mixture.
From brainly.in
the diagrams represent some elements mixtures and compounds which Is Pure Ice A Compound Element Or Mixture This is now a mixture of the elements iron and sulfur. A mixture is a material which has two or more types of molecules. The different elements are not joined together. If we take two or more pure substances and mix them together, we refer to this as a mixture. Ice is a compound, not a mixture. Mixtures can be. Is Pure Ice A Compound Element Or Mixture.
From quizlet.com
Elements, Compounds, and Mixtures Diagram Quizlet Is Pure Ice A Compound Element Or Mixture Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Ice is a compound, not a mixture. The different elements are not joined together. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. If we take two or more pure. Is Pure Ice A Compound Element Or Mixture.
From schematicpartebriose.z14.web.core.windows.net
Mixture Of Two Compounds Diagram Is Pure Ice A Compound Element Or Mixture Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected. Is Pure Ice A Compound Element Or Mixture.
From www.slideserve.com
PPT Matter and Change PowerPoint Presentation, free download ID2199820 Is Pure Ice A Compound Element Or Mixture When we mix two different pure substances together, like this, it’s a mixture. Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. This is now a mixture of the elements. Is Pure Ice A Compound Element Or Mixture.
From www.slideserve.com
PPT Elements, Compounds and Mixtures PowerPoint Presentation, free Is Pure Ice A Compound Element Or Mixture Mixtures can be classified as homogeneous or heterogeneous. Elements and compounds are both examples of pure substances. However, impure water creates ice that’s considered a. The different elements are not joined together. If we take two or more pure substances and mix them together, we refer to this as a mixture. Ice is a compound, not a mixture. A compound. Is Pure Ice A Compound Element Or Mixture.
From www.goodscience.com.au
Elements, Compounds and Mixtures Good Science Is Pure Ice A Compound Element Or Mixture Ice is water frozen into a solid state and comprises two elements — hydrogen and oxygen. A compound is a pure substance which contains identical molecules with two or more types of atomic core. Whereas a compound may have very different properties from the elements that compose it, in mixtures the substances keep their. When we mix two different pure. Is Pure Ice A Compound Element Or Mixture.
From manualworshipped.z14.web.core.windows.net
Diagram Of Mixture Of Elements Is Pure Ice A Compound Element Or Mixture This is now a mixture of the elements iron and sulfur. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. The different elements are not joined together. Mixtures can be classified as homogeneous or heterogeneous. Ice is water frozen into a solid state and. Is Pure Ice A Compound Element Or Mixture.
From www.numerade.com
SOLVED 10 POINTS!!!! Classify each as either element compound mixture Is Pure Ice A Compound Element Or Mixture The different elements are not joined together. However, impure water creates ice that’s considered a. If we take two or more pure substances and mix them together, we refer to this as a mixture. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Whereas a compound may have very. Is Pure Ice A Compound Element Or Mixture.
From temperaturemaster.com
What Category Does Dry Ice? Compound, Element, or Mixture Is Pure Ice A Compound Element Or Mixture For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Ice is a compound, not a mixture. A compound is a pure substance which contains identical molecules. Is Pure Ice A Compound Element Or Mixture.
From msjschemclass.blogspot.com
Ms J's Chemistry Class Matter! Is Pure Ice A Compound Element Or Mixture If we take two or more pure substances and mix them together, we refer to this as a mixture. Ice is a compound, not a mixture. Looking at the left side of the chart, we can see that a pure substance can be either an element or a compound. Whereas a compound may have very different properties from the elements. Is Pure Ice A Compound Element Or Mixture.
From www.thoughtco.com
What Are Examples of Pure Substances? Is Pure Ice A Compound Element Or Mixture This is now a mixture of the elements iron and sulfur. For example, we mentioned that oxygen is a compound as it consists of two oxygen atoms connected by covalent bonds. The different elements are not joined together. A mixture is a material which has two or more types of molecules. If we take two or more pure substances and. Is Pure Ice A Compound Element Or Mixture.
From manualworshipped.z14.web.core.windows.net
Element Compound Mixture Diagram Is Pure Ice A Compound Element Or Mixture Mixtures can be classified as homogeneous or heterogeneous. If we take two or more pure substances and mix them together, we refer to this as a mixture. Mixtures can always be separated again into component pure substances, because bonding among the atoms of the constituent substances does not occur in a mixture. Looking at the left side of the chart,. Is Pure Ice A Compound Element Or Mixture.