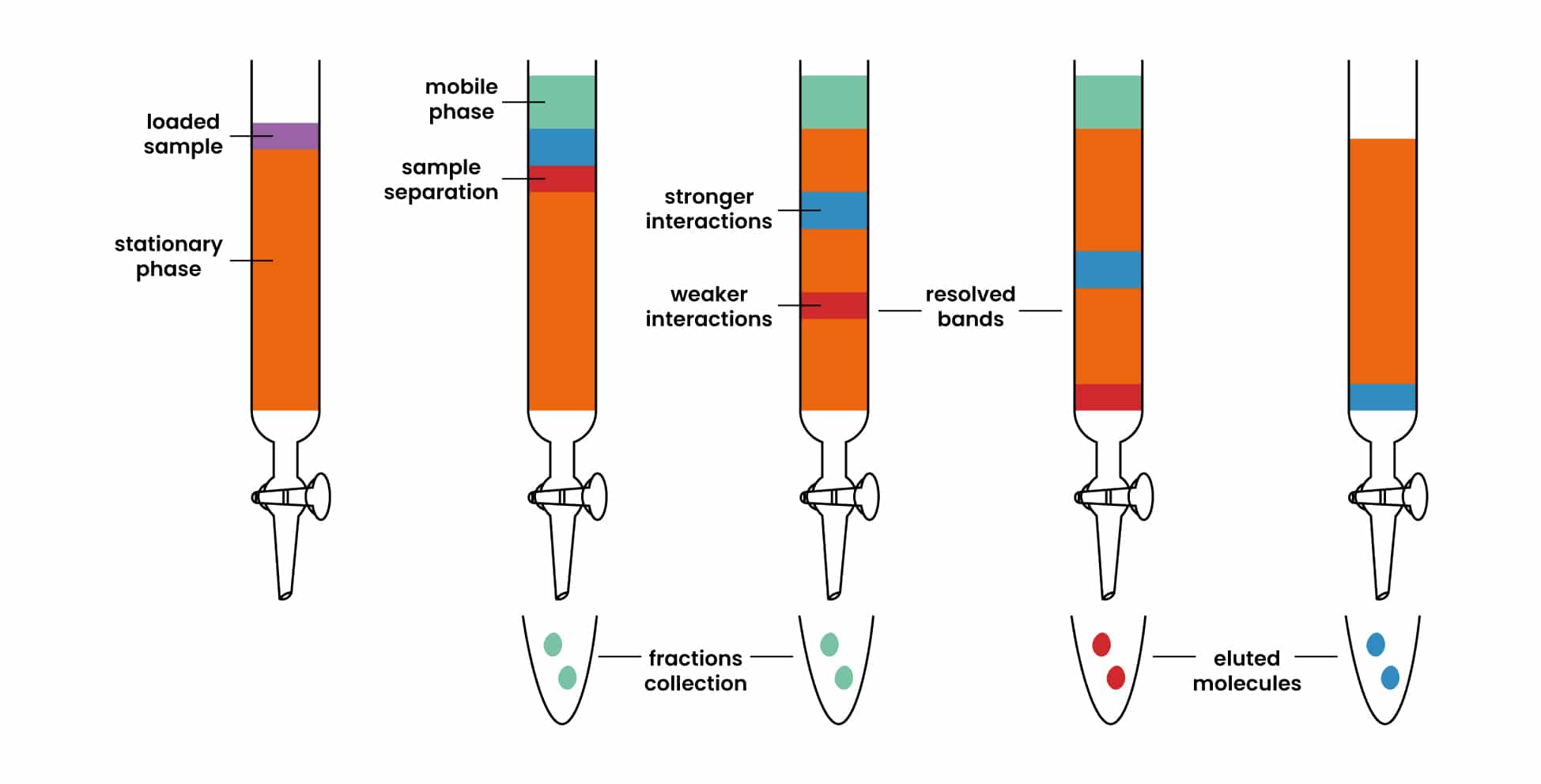
How Does Chromatography Work Chemistry . Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Specifically, differences in the chemical and physical properties of molecules. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. These are often coloured substances such as food. The fundamental principle separation in column chromatography relies on differences. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used.
from bitesizebio.com
The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. The fundamental principle separation in column chromatography relies on differences. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. These are often coloured substances such as food. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. Chromatography is a method by which a mixture is separated by distributing its components between two phases.
Column Chromatography Made Simple An Easy to Follow Guide
How Does Chromatography Work Chemistry Specifically, differences in the chemical and physical properties of molecules. Specifically, differences in the chemical and physical properties of molecules. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography is a separation technique used to separate mixtures of soluble substances. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. The fundamental principle separation in column chromatography relies on differences. These are often coloured substances such as food. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC How Does Chromatography Work Chemistry Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. These are often coloured substances such as food. The stationary phase remains fixed in place while the mobile phase. How Does Chromatography Work Chemistry.
From mavink.com
Thin Layer Chromatography Explained How Does Chromatography Work Chemistry Specifically, differences in the chemical and physical properties of molecules. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase acts as. How Does Chromatography Work Chemistry.
From www.nagwa.com
Lesson Video Chromatography Nagwa How Does Chromatography Work Chemistry The fundamental principle separation in column chromatography relies on differences. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. The stationary phase remains fixed in place while the mobile. How Does Chromatography Work Chemistry.
From www.youtube.com
Quick Introduction To Liquid Liquid Chromatography How Does It Work How Does Chromatography Work Chemistry Chromatography is a method by which a mixture is separated by distributing its components between two phases. The fundamental principle separation in column chromatography relies on differences. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is an important analytical. How Does Chromatography Work Chemistry.
From www.tes.com
AQA Chemistry Required Practical, Chromatography (6) Teaching Resources How Does Chromatography Work Chemistry Chromatography is a method by which a mixture is separated by distributing its components between two phases. The fundamental principle separation in column chromatography relies on differences. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting. How Does Chromatography Work Chemistry.
From travistra.blogspot.com
CHROMATOGRAPHY How Does Chromatography Work Chemistry The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. Chromatography is a separation technique used to separate mixtures of soluble substances. Specifically, differences in. How Does Chromatography Work Chemistry.
From microbeonline.com
Chromatography An Overview • Microbe Online How Does Chromatography Work Chemistry Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is a method by which a mixture is separated by distributing its components between two phases. These are. How Does Chromatography Work Chemistry.
From www.slideserve.com
PPT classification of chromatography PowerPoint Presentation, free How Does Chromatography Work Chemistry Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is a separation technique used to separate mixtures of soluble substances. The fundamental principle separation in column chromatography relies on differences. Chromatography is. How Does Chromatography Work Chemistry.
From www.rdworldonline.com
What is Gas Chromatography? Research & Development World How Does Chromatography Work Chemistry These are often coloured substances such as food. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. The stationary phase remains fixed in place while the mobile phase carries the components of the. How Does Chromatography Work Chemistry.
From www.slideserve.com
PPT Chromatography And Spectroscopy PowerPoint Presentation, free How Does Chromatography Work Chemistry Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is. How Does Chromatography Work Chemistry.
From khatiar1955a.altervista.org
Chromatography How Does Chromatography Work Chemistry Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase). How Does Chromatography Work Chemistry.
From www.youtube.com
Chromatography Organic Chemistry IIT JEE, NEET, CBSE YouTube How Does Chromatography Work Chemistry These are often coloured substances such as food. Specifically, differences in the chemical and physical properties of molecules. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is an important analytical technique because it allows chemists to separate substances. How Does Chromatography Work Chemistry.
From www.youtube.com
Basics of chromatography YouTube How Does Chromatography Work Chemistry Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. These are often coloured substances such as food.. How Does Chromatography Work Chemistry.
From collegedunia.com
Chromatography Principle, Types & Applications How Does Chromatography Work Chemistry The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Specifically, differences in the chemical and physical properties of molecules. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. Chromatography is an important analytical technique because it allows. How Does Chromatography Work Chemistry.
From www.pinterest.com
What Is Paper Chromatography and How Does it Work? High school How Does Chromatography Work Chemistry The fundamental principle separation in column chromatography relies on differences. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography, technique for separating the components, or solutes, of. How Does Chromatography Work Chemistry.
From www.learnatnoon.com
What is Chromatography? Noon Academy How Does Chromatography Work Chemistry The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. These are. How Does Chromatography Work Chemistry.
From www.youtube.com
Ion Exchange Chromatography Theory and Principle YouTube How Does Chromatography Work Chemistry Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The fundamental principle separation in column chromatography relies on differences. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute. How Does Chromatography Work Chemistry.
From keystagewiki.com
Chromatography Key Stage Wiki How Does Chromatography Work Chemistry Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. The stationary phase acts. How Does Chromatography Work Chemistry.
From collegedunia.com
Chromatography Principle, Types & Applications How Does Chromatography Work Chemistry Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is a separation technique used to separate mixtures of soluble substances. Specifically, differences in. How Does Chromatography Work Chemistry.
From www.youtube.com
Column chromatography principle and working YouTube How Does Chromatography Work Chemistry Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase). How Does Chromatography Work Chemistry.
From cezofmgw.blob.core.windows.net
Chromatography Principle Khan Academy at Clarence Odonnell blog How Does Chromatography Work Chemistry Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. Chromatography. How Does Chromatography Work Chemistry.
From microbenotes.com
14 Types of Chromatography (Definition, Principle, Steps, Uses) How Does Chromatography Work Chemistry Specifically, differences in the chemical and physical properties of molecules. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and. How Does Chromatography Work Chemistry.
From owlcation.com
What Is Paper Chromatography and How Does it Work? Owlcation How Does Chromatography Work Chemistry The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. The fundamental principle separation in column chromatography relies on differences. Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. Chromatography is a separation technique used to separate mixtures of soluble substances.. How Does Chromatography Work Chemistry.
From www.tffn.net
How Does Chromatography Work? A StepbyStep Guide to the Process and How Does Chromatography Work Chemistry These are often coloured substances such as food. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. The fundamental principle separation in column chromatography relies on differences. Specifically, differences in the chemical and physical. How Does Chromatography Work Chemistry.
From mavink.com
Chromatography Diagram Labeled How Does Chromatography Work Chemistry Specifically, differences in the chemical and physical properties of molecules. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is. How Does Chromatography Work Chemistry.
From www.bioanalysis-zone.com
What is chromatography and how does it work? Bioanalysis Zone How Does Chromatography Work Chemistry The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each. How Does Chromatography Work Chemistry.
From microbenotes.com
Thin Layer Chromatography Principle, Parts, Steps, Uses How Does Chromatography Work Chemistry Chromatography is an important analytical technique because it allows chemists to separate substances in complex mixtures. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed. How Does Chromatography Work Chemistry.
From scienceinfo.com
Ion Exchange Chromatography Principle, Types, Procedure, Applications How Does Chromatography Work Chemistry Specifically, differences in the chemical and physical properties of molecules. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them. How Does Chromatography Work Chemistry.
From materialmcgheeriveted.z21.web.core.windows.net
Plant Pigment Chromatography Lab Report How Does Chromatography Work Chemistry The fundamental principle separation in column chromatography relies on differences. Specifically, differences in the chemical and physical properties of molecules. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Chromatography is an important. How Does Chromatography Work Chemistry.
From www.chemistrystudent.com
Chromatography (ALevel) ChemistryStudent How Does Chromatography Work Chemistry Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving fluid stream, called the mobile phase, and a contiguous stationary phase. Specifically, differences in the chemical and physical properties of molecules. The stationary. How Does Chromatography Work Chemistry.
From www.priyamstudycentre.com
High Performance Liquid Chromatography (HPLC) Principle How Does Chromatography Work Chemistry The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. These are often coloured substances such as food. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is. How Does Chromatography Work Chemistry.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide How Does Chromatography Work Chemistry These are often coloured substances such as food. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid. How Does Chromatography Work Chemistry.
From www.youtube.com
Paper Chromatography Chemistry Experiment Chemistry Experiment with How Does Chromatography Work Chemistry The fundamental principle separation in column chromatography relies on differences. Specifically, differences in the chemical and physical properties of molecules. Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each. Chromatography is a separation technique used to separate mixtures of soluble substances.. How Does Chromatography Work Chemistry.
From mavink.com
Chromatography Diagram Labeled How Does Chromatography Work Chemistry Chromatography is actually a way of separating out a mixture of chemicals, which are in gas or liquid form, by letting them creep. Specifically, differences in the chemical and physical properties of molecules. Chromatography is a separation technique used to separate mixtures of soluble substances. The fundamental principle separation in column chromatography relies on differences. Chromatography is a method by. How Does Chromatography Work Chemistry.
From www.bioanalysis-zone.com
What is chromatography and how does it work? Bioanalysis Zone How Does Chromatography Work Chemistry The stationary phase acts as a constraint on many of the components in a mixture, slowing them down. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography, technique for separating the components, or solutes, of a mixture on the basis of the relative amounts of each solute distributed between a moving. How Does Chromatography Work Chemistry.