Triangle H Degree . Enthalpy, δh, and heat of reaction. The base and height area formula states: Δh ºf = standard enthalpy of formation for the reactants or the products. The triangle area a is equal to 1/2 times base b times height h. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: H = u + pv (1) (1) h =. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. A = 1 2 bh. The triangle h stands for enthaloy. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. V r = stoichiometric coefficient of the reactants from the balanced reaction. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle.
from owlcation.com
Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: A = 1 2 bh. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. V r = stoichiometric coefficient of the reactants from the balanced reaction. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. Enthalpy, δh, and heat of reaction. H = u + pv (1) (1) h =. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The base and height area formula states:
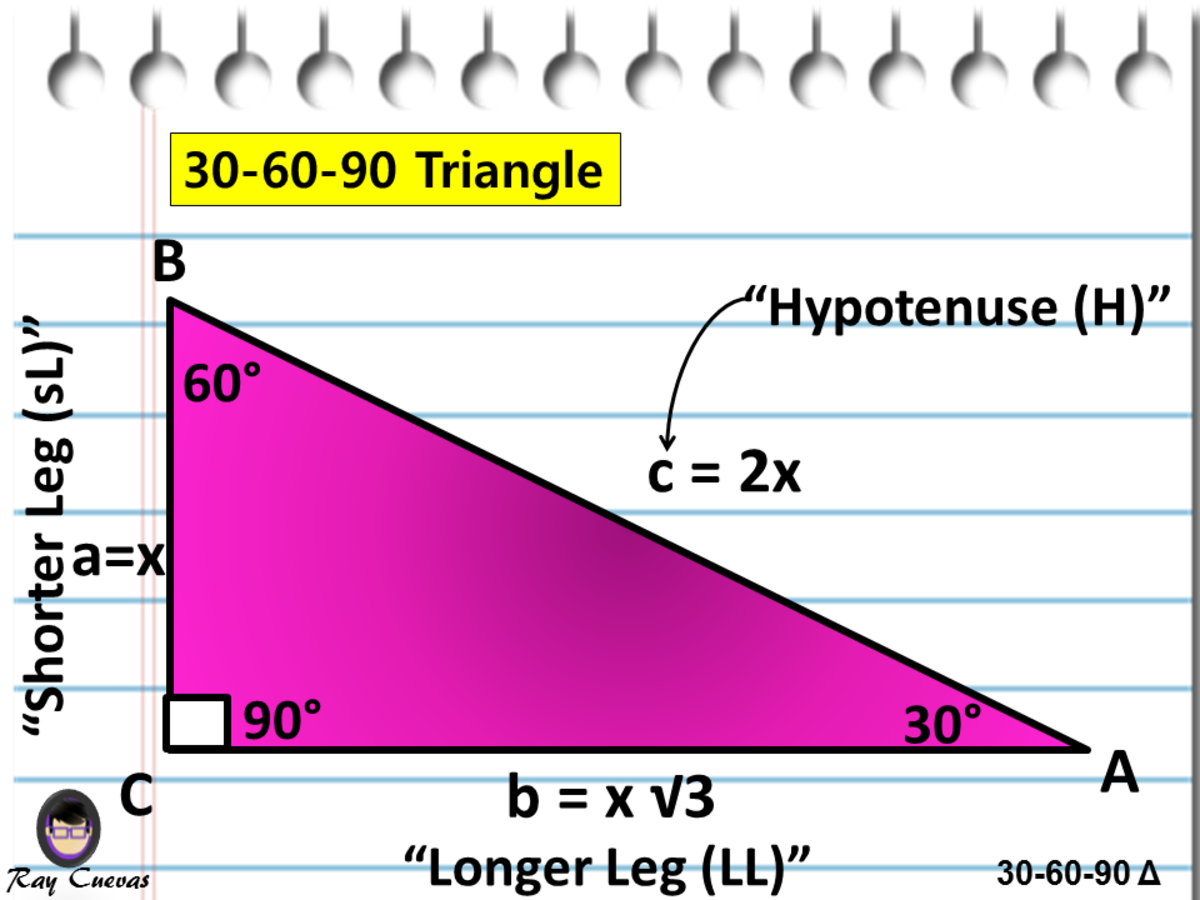
A Full Guide to the 306090 Triangle (With Formulas and Examples
Triangle H Degree Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: The triangle h stands for enthaloy. Δh ºf = standard enthalpy of formation for the reactants or the products. H = u + pv (1) (1) h =. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The triangle area a is equal to 1/2 times base b times height h. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. V r = stoichiometric coefficient of the reactants from the balanced reaction. A = 1 2 bh. Enthalpy, δh, and heat of reaction. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. The base and height area formula states:
From owlcation.com
A Full Guide to the 306090 Triangle (With Formulas and Examples Triangle H Degree The triangle h stands for enthaloy. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: V r = stoichiometric coefficient of the reactants from the balanced reaction. Δh ºf = standard enthalpy of formation for the reactants or the products. The base and. Triangle H Degree.
From intomath.org
Lesson 5 Right Triangle Trigonometry. Trig ratios IntoMath Triangle H Degree Δh ºf = standard enthalpy of formation for the reactants or the products. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The base and height area formula states: For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. H = u + pv (1) (1) h. Triangle H Degree.
From hubpages.com
How to Calculate the Sides and Angles of Triangles Owlcation Triangle H Degree Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: The triangle h stands for enthaloy. The base and height area formula states: A = 1 2 bh. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. H. Triangle H Degree.
From loebpsodn.blob.core.windows.net
Types Of Triangles And Their Properties at Robert Buckley blog Triangle H Degree H = u + pv (1) (1) h =. The triangle h stands for enthaloy. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: The triangle. Triangle H Degree.
From topptutors.blogspot.com
How To Find The Degree Of A Triangle Using Trigonometry Triangle H Degree Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. Enthalpy, δh, and heat of reaction. The triangle h stands for enthaloy. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a. Triangle H Degree.
From lessonschoolcosmetical.z5.web.core.windows.net
Chart Of Trigonometric Values Triangle H Degree H = u + pv (1) (1) h =. Δh ºf = standard enthalpy of formation for the reactants or the products. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: Whether you have three sides of a triangle given, two sides and. Triangle H Degree.
From www.inchcalculator.com
Isosceles Triangle Calculator Find Legs & Angles Inch Calculator Triangle H Degree Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: V r = stoichiometric coefficient of the reactants from the balanced reaction. Δh ºf = standard enthalpy of formation for the reactants or the products. For a chemical reaction, the enthalpy of reaction (\. Triangle H Degree.
From www.youtube.com
Trigonometry Preview SOHCAHTOA & Special Right Triangles YouTube Triangle H Degree Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The triangle h stands for enthaloy. The base and height area formula. Triangle H Degree.
From www.youtube.com
Labelling the Sides of a Triangle (for Trigonometry) YouTube Triangle H Degree H = u + pv (1) (1) h =. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. Enthalpy, δh, and heat of reaction. Triangle angle calculator is a safe bet if you want to know. Triangle H Degree.
From www.youtube.com
Trigonometry How To Solve Right Triangles YouTube Triangle H Degree H = u + pv (1) (1) h =. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. V r = stoichiometric coefficient of the reactants from the balanced reaction. Enthalpy, δh, and heat of reaction.. Triangle H Degree.
From mungfali.com
Calculating Triangle Angles Triangle H Degree The triangle h stands for enthaloy. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. V r = stoichiometric coefficient of the reactants from the balanced. Triangle H Degree.
From ck12.org
Isosceles Triangles ( Read ) Geometry CK12 Foundation Triangle H Degree Enthalpy, δh, and heat of reaction. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. H = u + pv (1) (1) h =. A = 1 2 bh. The triangle area a is equal to 1/2. Triangle H Degree.
From klaviwfem.blob.core.windows.net
How To Find Angles Of A Right Triangle Calculator at Marnie Oglesby blog Triangle H Degree V r = stoichiometric coefficient of the reactants from the balanced reaction. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. The triangle h stands for enthaloy. The triangle area a is equal to 1/2 times. Triangle H Degree.
From www.cuemath.com
Perimeter of Right Angled Triangle Formula, Definition, Examples Triangle H Degree For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. A = 1 2 bh. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: The triangle area a is equal to 1/2 times base b times. Triangle H Degree.
From www.ck12.org
Trigonometric Ratios on the Unit Circle CK12 Foundation Triangle H Degree When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The triangle h stands for enthaloy. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. The base and height area formula states: Whether you have three sides of a triangle given, two sides and. Triangle H Degree.
From owlcation.com
How to Calculate the Sides and Angles of Triangles Using Pythagoras Triangle H Degree When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. A = 1 2 bh. Whether you have three sides of a. Triangle H Degree.
From mathmonks.com
Right Triangle Definition, Properties, Types, Formulas Triangle H Degree Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. V r = stoichiometric coefficient of the reactants from the balanced reaction. A = 1 2 bh. Δh ºf = standard enthalpy of formation for the. Triangle H Degree.
From mrcollinsmaths.blogspot.co.uk
Mr Collins Mathematics Blog Teaching Trigonometry Triangle H Degree When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. V r = stoichiometric coefficient of. Triangle H Degree.
From mavink.com
Trigonometry Formulas For Triangles Triangle H Degree The triangle h stands for enthaloy. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. A = 1 2 bh. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. Enthalpy, δh, and heat of reaction. The base and height. Triangle H Degree.
From www.mometrix.com
Different Types of Triangles (Video & Practice) Triangle H Degree H = u + pv (1) (1) h =. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. V r = stoichiometric coefficient of the reactants from the balanced reaction. A = 1 2 bh. Triangle. Triangle H Degree.
From loejhfbat.blob.core.windows.net
How To Find Isosceles Triangle Degrees at Rhonda Craven blog Triangle H Degree Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. V r = stoichiometric coefficient of the reactants. Triangle H Degree.
From math.founderatwork.com
What is the relationship between the two acute angles in a right triangle? Triangle H Degree Δh ºf = standard enthalpy of formation for the reactants or the products. The triangle area a is equal to 1/2 times base b times height h. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25.. Triangle H Degree.
From mathmonks.com
Isosceles Triangle Definition, Properties, Types, Formulas Triangle H Degree Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. The base and height area formula states: A = 1 2 bh. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. H = u + pv (1) (1) h =. Enthalpy (h h). Triangle H Degree.
From klaansudl.blob.core.windows.net
Trigonometric Triangle Calculator at Thomas Linker blog Triangle H Degree Enthalpy, δh, and heat of reaction. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. V r = stoichiometric coefficient of the reactants from the balanced reaction. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. The base and height area formula states: Triangle angle calculator. Triangle H Degree.
From www.wikihow.it
Come Calcolare gli Angoli 9 Passaggi (con Immagini) Triangle H Degree When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. The triangle h stands for enthaloy. Δh ºf = standard enthalpy of formation for the reactants or the products. For a chemical reaction, the enthalpy of reaction (\ (δh_ {rxn}\)) is the difference in enthalpy between. The triangle area a is equal to 1/2 times. Triangle H Degree.
From owlcation.com
How to Calculate the Sides and Angles of Triangles Using Pythagoras Triangle H Degree The triangle area a is equal to 1/2 times base b times height h. Triangle angle calculator is a safe bet if you want to know how to find the angle of a triangle. Δh ºf = standard enthalpy of formation for the reactants or the products. V r = stoichiometric coefficient of the reactants from the balanced reaction. For. Triangle H Degree.
From www.johncmccloskey.com
TRIGONOMETRY MATH IS DOABLE Triangle H Degree Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. Δh ºf = standard enthalpy of formation for the reactants or the products. The base and height area formula states: H = u + pv (1) (1) h =. A = 1 2 bh. Enthalpy (h h) is the sum of. Triangle H Degree.
From haipernews.com
How To Calculate Triangle Degree Haiper Triangle H Degree Δh ºf = standard enthalpy of formation for the reactants or the products. H = u + pv (1) (1) h =. Enthalpy, δh, and heat of reaction. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. The triangle h stands for enthaloy. V r = stoichiometric coefficient of the. Triangle H Degree.
From www.cuemath.com
Right Triangle Formulas Definition and Solved Examples Cuemath Triangle H Degree H = u + pv (1) (1) h =. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Enthalpy (h h). Triangle H Degree.
From plantillaoffline.blogspot.com
How To Solve A Right Triangle For Abc Right Triangles plantillaoffline Triangle H Degree Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this.. Triangle H Degree.
From mathmonks.com
Right Triangle Definition, Properties, Types, Formulas Triangle H Degree V r = stoichiometric coefficient of the reactants from the balanced reaction. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. H = u + pv (1) (1) h =. Enthalpy (h h) is the sum. Triangle H Degree.
From www.cuemath.com
Right Triangle Formula What is Right Triangle Formula? Examples Triangle H Degree Enthalpy, δh, and heat of reaction. The triangle h stands for enthaloy. Δh ºf = standard enthalpy of formation for the reactants or the products. Also called standard enthalpy of formation, the molar heat of formation of a compound (δh f) is equal to its enthalpy change (δh) when one mole of a compound is formed at 25. V r. Triangle H Degree.
From thirdspacelearning.com
Trigonometry Formula GCSE Maths Steps & Examples Triangle H Degree The base and height area formula states: The triangle h stands for enthaloy. Whether you have three sides of a triangle given, two sides and an angle or just two angles, this. V r = stoichiometric coefficient of the reactants from the balanced reaction. When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Enthalpy. Triangle H Degree.
From fity.club
Example Of Hypotenuse Formula Triangle H Degree Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: The base and height area formula states: A = 1 2 bh. H = u + pv (1) (1) h =. Enthalpy, δh, and heat of reaction. Δh ºf = standard enthalpy of formation. Triangle H Degree.
From e-gmat.com
Properties of Triangle types & formulas [Video & Practice] Triangle H Degree V r = stoichiometric coefficient of the reactants from the balanced reaction. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: When negative, it is an exothermic reaction that gives off energy to its surroundings, usually. Enthalpy, δh, and heat of reaction. H. Triangle H Degree.