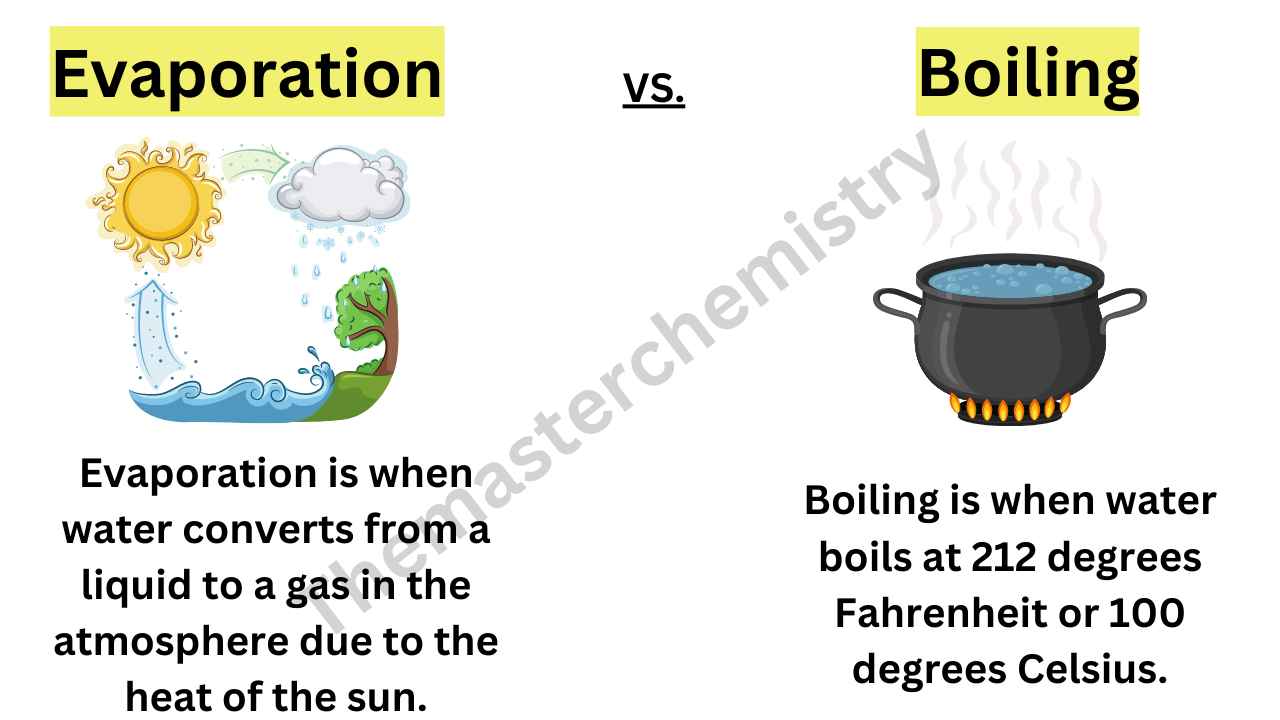
Boiling And Evaporation Temperature . This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. It occurs when the liquid reaches a. Boiling is the process of converting a liquid into vapor by heating it to its boiling point.
from themasterchemistry.com
It occurs when the liquid reaches a. Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the.
Difference Between Evaporation And BoilingEvaporation Vs Boiling
Boiling And Evaporation Temperature Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. It occurs when the liquid reaches a. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any.
From www.scribd.com
Differences Between and Evaporation Boiling PDF Boiling And Evaporation Temperature Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling occurs at the boiling point and is characterized by. It occurs when the liquid reaches a. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas.. Boiling And Evaporation Temperature.
From exyukbtsg.blob.core.windows.net
Is Boiling Water Evaporation at Lawrence Edwards blog Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. It occurs when the liquid reaches a. Boiling is the process of. Boiling And Evaporation Temperature.
From brunofuga.adv.br
What Is The Difference Between Boiling And Evaporation, 50 OFF Boiling And Evaporation Temperature Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling occurs at the boiling point and is characterized by. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature. Boiling And Evaporation Temperature.
From www.toppr.com
Differences Between Evaporation and Boiling in Chemistry Boiling And Evaporation Temperature It occurs when the liquid reaches a. Boiling occurs at the boiling point and is characterized by. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid. Boiling And Evaporation Temperature.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Boiling And Evaporation Temperature Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs at the. Boiling And Evaporation Temperature.
From klafvmutm.blob.core.windows.net
Heat Source For Evaporation at Salvatore Shorey blog Boiling And Evaporation Temperature Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. It occurs when the liquid reaches a. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling occurs at the boiling point and is characterized by. Boiling is the process. Boiling And Evaporation Temperature.
From www.chegg.com
Solved Example Calculate the evaporation rate from an open Boiling And Evaporation Temperature It occurs when the liquid reaches a. Boiling occurs at the boiling point and is characterized by. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes. Boiling And Evaporation Temperature.
From www.chegg.com
Solved The Boiling Temperature Of Oxygen At Atmospheric P... Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. It occurs when the liquid reaches a. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the.. Boiling And Evaporation Temperature.
From www.theengineeringconcepts.com
Difference between Boiling & Evaporation. The Engineering Concepts Boiling And Evaporation Temperature Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling and evaporation are distinct processes that involve the conversion. Boiling And Evaporation Temperature.
From www.diffzy.com
Evaporation vs Boiling What's the Difference (With Table) Boiling And Evaporation Temperature Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling occurs at the boiling point and is characterized by. Boiling, on the other hand, refers to the process of. Boiling And Evaporation Temperature.
From themasterchemistry.com
Difference Between Evaporation And BoilingEvaporation Vs Boiling Boiling And Evaporation Temperature Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas.. Boiling And Evaporation Temperature.
From www.teachoo.com
What is the Difference between Evaporation and Boiling? Class 9 Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. This causes an increase in temperature when the material is. Boiling And Evaporation Temperature.
From www.youtube.com
Difference between Evaporation and Boiling science learning academy Boiling And Evaporation Temperature This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs throughout the entire. Boiling And Evaporation Temperature.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Boiling And Evaporation Temperature Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a. Boiling And Evaporation Temperature.
From www.shutterstock.com
Vektor Stok Evaporation Boiling Point Water Vector (Tanpa Royalti Boiling And Evaporation Temperature It occurs when the liquid reaches a. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling and evaporation are distinct processes that involve the. Boiling And Evaporation Temperature.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Boiling And Evaporation Temperature Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place. Boiling And Evaporation Temperature.
From thecontentauthority.com
Boiling vs Evaporation Which One Is The Correct One? Boiling And Evaporation Temperature This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling is the process of converting a liquid into vapor by. Boiling And Evaporation Temperature.
From pediaa.com
Difference Between Evaporation and Boiling Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. It occurs when the liquid reaches a. This causes an increase in temperature when the material is. Boiling And Evaporation Temperature.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Boiling And Evaporation Temperature It occurs when the liquid reaches a. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling, on the other hand, refers to the process of heating up a liquid where. Boiling And Evaporation Temperature.
From physics.stackexchange.com
thermodynamics Evaporation of water at room temperature Physics Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the.. Boiling And Evaporation Temperature.
From www.merchantnavydecoded.com
What is Evaporation and boiling? Merchant Navy Decoded Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling and evaporation are distinct processes. Boiling And Evaporation Temperature.
From www.youtube.com
Evaporation vs Boiling Point YouTube Boiling And Evaporation Temperature Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas.. Boiling And Evaporation Temperature.
From www.pinterest.com
evaporation vs boiling Boiling And Evaporation Temperature Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs throughout the entire volume of the liquid at a. Boiling And Evaporation Temperature.
From ar.inspiredpencil.com
Evaporation Examples Boiling And Evaporation Temperature This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling, on the other hand, refers to the process of heating up. Boiling And Evaporation Temperature.
From exolwxybp.blob.core.windows.net
Liquid Hydrogen Temperature And Pressure at Martha Merrick blog Boiling And Evaporation Temperature Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. It occurs when the. Boiling And Evaporation Temperature.
From 88guru.com
Difference Between Evaporation and Boiling Boiling And Evaporation Temperature It occurs when the liquid reaches a. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs at the boiling point and is characterized by. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other. Boiling And Evaporation Temperature.
From www.slideserve.com
PPT Evaporation and Boiling Point PowerPoint Presentation ID2792222 Boiling And Evaporation Temperature It occurs when the liquid reaches a. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid. Boiling And Evaporation Temperature.
From www.diffzy.com
Evaporation vs Boiling What's the Difference (With Table) Boiling And Evaporation Temperature This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. Boiling is the process of converting a liquid into vapor by. Boiling And Evaporation Temperature.
From exyukbtsg.blob.core.windows.net
Is Boiling Water Evaporation at Lawrence Edwards blog Boiling And Evaporation Temperature Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being. Boiling And Evaporation Temperature.
From www.congress-intercultural.eu
Dmso Boiling Point Deals www.congressintercultural.eu Boiling And Evaporation Temperature This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being. Boiling And Evaporation Temperature.
From stock.adobe.com
Water boiling, freezing, melting degrees. Evaporation. . Liquids Boiling And Evaporation Temperature It occurs when the liquid reaches a. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs at the boiling point and is characterized by. Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface. Boiling And Evaporation Temperature.
From 88guru.com
Difference Between Evaporation and Boiling Boiling And Evaporation Temperature Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. It occurs when the liquid reaches a. This causes an increase in temperature when the material is a solid, liquid or. Boiling And Evaporation Temperature.
From chart-studio.plotly.com
Temperature vs. Time of Evaporation of Fluids scatter chart made by K Boiling And Evaporation Temperature Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling occurs at the boiling point and is characterized by. Boiling and evaporation are distinct processes that involve the conversion of a liquid into a gas. It occurs when the liquid reaches a. Boiling, on the other hand, refers to the process of. Boiling And Evaporation Temperature.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Boiling And Evaporation Temperature Boiling occurs throughout the entire volume of the liquid at a specific temperature, while evaporation takes place at the liquid's surface at any. Boiling occurs at the boiling point and is characterized by. It occurs when the liquid reaches a. Boiling is the process of converting a liquid into vapor by heating it to its boiling point. Boiling and evaporation. Boiling And Evaporation Temperature.
From www.youtube.com
Difference between Boiling and Evaporation Class 8th jatinacademy Boiling And Evaporation Temperature Boiling, on the other hand, refers to the process of heating up a liquid where the temperature of the liquid being heated up is greater than the. This causes an increase in temperature when the material is a solid, liquid or gas, but does not cause an increase in. Boiling occurs throughout the entire volume of the liquid at a. Boiling And Evaporation Temperature.