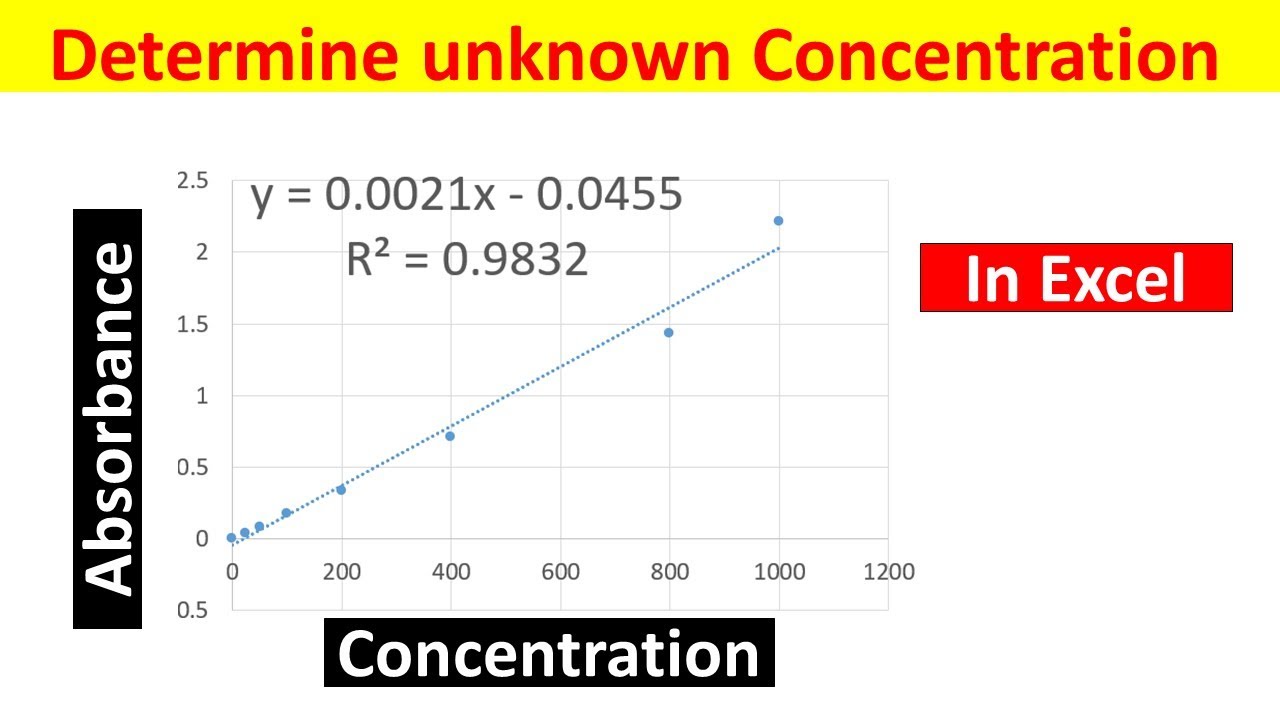
Concentration Of An Unknown Solution . Calculate the concentration of ions in a soluble ionic compound. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. By locating the absorbance of the unknown on the vertical axis of the graph, the. Data collected during a titration allows chemists to determine a solution’s unknown concentration. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Concordant titres should be used when calculating. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. A concentrated solution is one that has a relatively large amount of dissolved solute. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry.
from www.youtube.com
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. By locating the absorbance of the unknown on the vertical axis of the graph, the. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Concordant titres should be used when calculating. Calculate the concentration of ions in a soluble ionic compound. Data collected during a titration allows chemists to determine a solution’s unknown concentration. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. A concentrated solution is one that has a relatively large amount of dissolved solute.
Generating Standard Curve and Determining Concentration of Unknown
Concentration Of An Unknown Solution A concentrated solution is one that has a relatively large amount of dissolved solute. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. A concentrated solution is one that has a relatively large amount of dissolved solute. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. By locating the absorbance of the unknown on the vertical axis of the graph, the. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Calculate the concentration of ions in a soluble ionic compound. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Concordant titres should be used when calculating. Data collected during a titration allows chemists to determine a solution’s unknown concentration.
From www.chegg.com
Solved 3. Calculate the concentration of unknown "I using Concentration Of An Unknown Solution Calculate the concentration of ions in a soluble ionic compound. By locating the absorbance of the unknown on the vertical axis of the graph, the. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Concordant titres should be used when calculating. The concentration of a solution is a measure of the amount. Concentration Of An Unknown Solution.
From www.youtube.com
The titration of a 10 00 mL sample of HCL solution of unknown Concentration Of An Unknown Solution By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Data collected during a titration allows chemists to determine a solution’s unknown concentration. By knowing the volume and concentration of. Concentration Of An Unknown Solution.
From www.chegg.com
Solved 30.00 mL of a H2SO4 solution with an unknown Concentration Of An Unknown Solution By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. A concentrated solution is one that has a relatively large amount of dissolved solute. Outline the steps to make a solution of a desired concentration from a. Concentration Of An Unknown Solution.
From www.numerade.com
SOLVED A 28.00 mL sample of an H2SO4 solution of unknown concentration Concentration Of An Unknown Solution By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. A concentrated solution is one that has a relatively large amount of. Concentration Of An Unknown Solution.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Concentration Of An Unknown Solution The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Concordant titres should be used when calculating. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. By knowing the volume and concentration. Concentration Of An Unknown Solution.
From www.coursehero.com
[Solved] Problem What is the molar concentration of an unknown sodium Concentration Of An Unknown Solution By locating the absorbance of the unknown on the vertical axis of the graph, the. Concordant titres should be used when calculating. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution.. Concentration Of An Unknown Solution.
From askfilo.com
उ० An aqueous solution contains an unknown concentration of Ba2+ When 50 Concentration Of An Unknown Solution Data collected during a titration allows chemists to determine a solution’s unknown concentration. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. By locating the. Concentration Of An Unknown Solution.
From www.chegg.com
Solved At the bottom it says " Enter the concentration of Concentration Of An Unknown Solution What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Calculate the concentration of ions in a soluble ionic compound. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Data collected. Concentration Of An Unknown Solution.
From www.coursehero.com
[Solved] The concentration of an unknown sample of sulfuric acid was Concentration Of An Unknown Solution Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Calculate the concentration of ions in a soluble ionic compound. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. The concentration of a solution is a measure of the amount. Concentration Of An Unknown Solution.
From www.chegg.com
Solved A NaOH solution of unknown concentration is titrated Concentration Of An Unknown Solution The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Data collected during a titration allows chemists to determine a solution’s unknown concentration. By knowing the volume and concentration of. Concentration Of An Unknown Solution.
From www.studypool.com
SOLUTION Determination of the concentration of an unknown kmno4 Concentration Of An Unknown Solution Data collected during a titration allows chemists to determine a solution’s unknown concentration. A concentrated solution is one that has a relatively large amount of dissolved solute. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. What if the concentration of an unknown is. Concentration Of An Unknown Solution.
From www.youtube.com
Generating Standard Curve and Determining Concentration of Unknown Concentration Of An Unknown Solution Data collected during a titration allows chemists to determine a solution’s unknown concentration. By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. The concentration of a solution is a measure of the amount of solute that. Concentration Of An Unknown Solution.
From www.bartleby.com
Answered 2) If an unknown Cu*2 solution has an… bartleby Concentration Of An Unknown Solution Calculate the concentration of ions in a soluble ionic compound. By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Concordant titres should be used when calculating. By knowing the volume and concentration of the standardized solution. Concentration Of An Unknown Solution.
From www.chegg.com
Solved Calculating concentration of an unknown using beer Concentration Of An Unknown Solution Concordant titres should be used when calculating. A concentrated solution is one that has a relatively large amount of dissolved solute. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. The concentration of a solution is a measure of the amount of solute that has been dissolved. Concentration Of An Unknown Solution.
From www.toppr.com
The molal elevation of an unknown solution is equal to the molal Concentration Of An Unknown Solution By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Data collected during a titration allows chemists to determine a solution’s unknown concentration. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption. Concentration Of An Unknown Solution.
From www.numerade.com
SOLVED The flask contains 25 mL of an unknown diprotic acid aqueous Concentration Of An Unknown Solution A concentrated solution is one that has a relatively large amount of dissolved solute. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. Calculate the concentration of. Concentration Of An Unknown Solution.
From www.chegg.com
Solved 25.00 mL of a H2SO4 solution with an unknown Concentration Of An Unknown Solution The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Calculate the concentration of ions in a soluble ionic compound. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. By locating. Concentration Of An Unknown Solution.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Concentration Of An Unknown Solution What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution. Concentration Of An Unknown Solution.
From brainly.in
How to find the concentration of an unknown solution using a standard Concentration Of An Unknown Solution The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Calculate the concentration of ions in a soluble ionic compound. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the. Concentration Of An Unknown Solution.
From www.chegg.com
Solved Given the following information (in photos), how do I Concentration Of An Unknown Solution Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. A concentrated solution is one that has a relatively large amount of dissolved solute. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Calculate the concentration. Concentration Of An Unknown Solution.
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube Concentration Of An Unknown Solution The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Concordant titres should be used when calculating. Outline the steps to make a. Concentration Of An Unknown Solution.
From www.chegg.com
Solved Determine the concentration of an unknown solution Concentration Of An Unknown Solution Calculate the concentration of ions in a soluble ionic compound. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. By locating the absorbance of the unknown. Concentration Of An Unknown Solution.
From www.studypool.com
SOLUTION Titration theory titration is a laboratory technique by which Concentration Of An Unknown Solution By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. What if the concentration of an unknown is higher than the range of concentration used when you. Concentration Of An Unknown Solution.
From www.studypool.com
SOLUTION Determination of the concentration of an unknown kmno4 Concentration Of An Unknown Solution By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. Outline the steps. Concentration Of An Unknown Solution.
From www.youtube.com
AcidBase Titrations Calculating Concentration of a Standard Solution Concentration Of An Unknown Solution By locating the absorbance of the unknown on the vertical axis of the graph, the. Data collected during a titration allows chemists to determine a solution’s unknown concentration. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. A concentrated solution is one that has a relatively large amount of dissolved solute. By. Concentration Of An Unknown Solution.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide Concentration Of An Unknown Solution Calculate the concentration of ions in a soluble ionic compound. A concentrated solution is one that has a relatively large amount of dissolved solute. Data collected during a titration allows chemists to determine a solution’s unknown concentration. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. The. Concentration Of An Unknown Solution.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Concentration Of An Unknown Solution The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Calculate the concentration of ions in a soluble ionic. Concentration Of An Unknown Solution.
From www.numerade.com
SOLVEDA 25.00\mathrm{mL} sample of an \mathrm{H}_{2} \mathrm{SO}_{4 Concentration Of An Unknown Solution The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. By locating the absorbance of the unknown on the vertical axis of the graph, the. Data collected during a titration allows chemists to determine a solution’s unknown concentration. A concentrated solution is one that has. Concentration Of An Unknown Solution.
From www.researchgate.net
How can I calculate concentration of solution by using UV spectrometer Concentration Of An Unknown Solution Concordant titres should be used when calculating. By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. The concentration of an unknown. Concentration Of An Unknown Solution.
From www.youtube.com
[Example] How to Find the Concentration of a Solution. YouTube Concentration Of An Unknown Solution By knowing the volume and concentration of the standardized solution added to the unknown solution, the concentration of the unknown solution can be calculated using stoichiometry. Concordant titres should be used when calculating. By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of an unknown niso 4 solution is then determined by. Concentration Of An Unknown Solution.
From www.chegg.com
Solved Determination of unknown concentration of an acid or Concentration Of An Unknown Solution Calculate the concentration of ions in a soluble ionic compound. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. A concentrated solution is one that has a relatively large amount of dissolved solute. What if the concentration of an unknown is higher than the. Concentration Of An Unknown Solution.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Concentration Of An Unknown Solution By locating the absorbance of the unknown on the vertical axis of the graph, the. Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. A concentrated solution is one that has a relatively large amount of dissolved solute. Concordant titres should be used when calculating. What if the concentration of an unknown. Concentration Of An Unknown Solution.
From www.chegg.com
Solved A beaker contains a 25 mL solution of an unknown Concentration Of An Unknown Solution The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. The concentration of an unknown niso 4 solution is then determined by measuring. Concentration Of An Unknown Solution.
From www.chegg.com
Solved You titrate 25.0 mL of HCl Concentration Of An Unknown Solution What if the concentration of an unknown is higher than the range of concentration used when you were doing an absorption v. Data collected during a titration allows chemists to determine a solution’s unknown concentration. The concentration of an unknown niso 4 solution is then determined by measuring its absorbance with the colorimeter. By locating the absorbance of the unknown. Concentration Of An Unknown Solution.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution Concentration Of An Unknown Solution Outline the steps to make a solution of a desired concentration from a solid or aqueous solute. By locating the absorbance of the unknown on the vertical axis of the graph, the. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. By knowing the. Concentration Of An Unknown Solution.