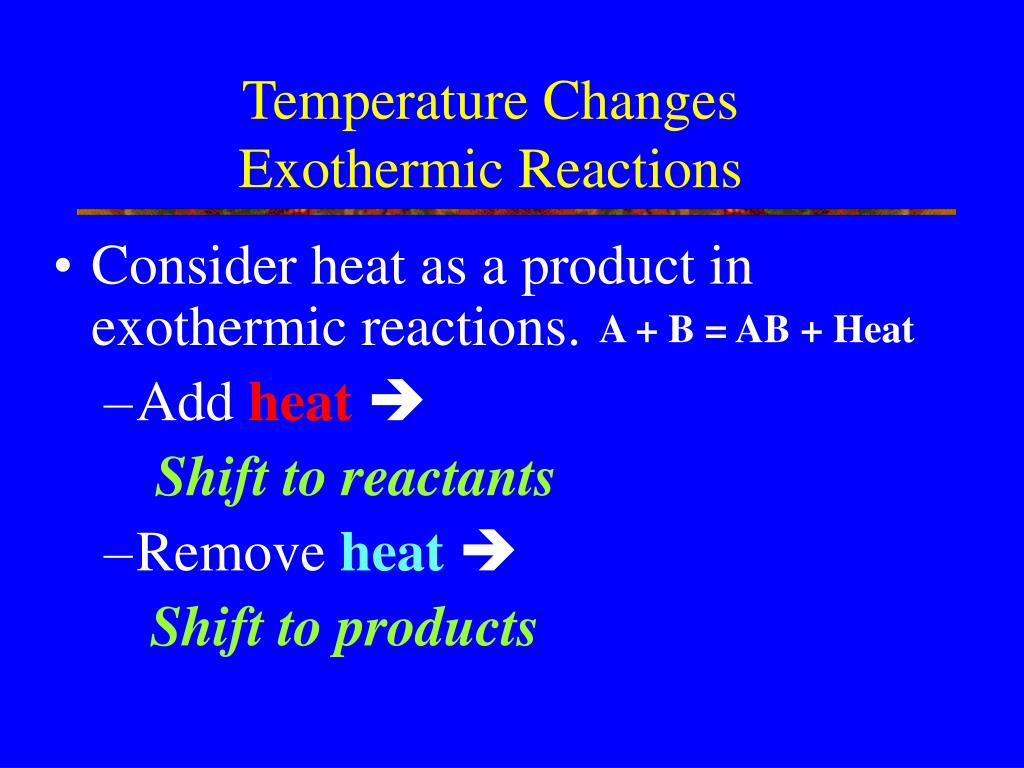
How Does Temperature Change Kc . But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! If δh is positive, reaction is endothermic, then: Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. For an endothermic reaction such as: The equilibrium constant is only affected by temperature. A temperature change occurs when temperature is increased or decreased by the flow of heat. Equilibrium constants are changed if you change the temperature of the system. [h 2] and [i 2]. For exothermic and endothermic reactions, this added stress is a change in temperature. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. It does not change if concentrations or pressures. Look at the equilibrium involving. This shifts chemical equilibria toward the. The effect of temperature on kc and kp. Changes in temperature change the equilibrium constant kc.
from www.slideserve.com
But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For an endothermic reaction such as: [h 2] and [i 2]. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. It does not change if concentrations or pressures. If δh is positive, reaction is endothermic, then: Equilibrium constants are changed if you change the temperature of the system. K c or k p are constant at constant temperature, but they vary as the temperature changes. A temperature change occurs when temperature is increased or decreased by the flow of heat.
PPT Le Chatelier’s Principle PowerPoint Presentation, free download
How Does Temperature Change Kc But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! If δh is positive, reaction is endothermic, then: A temperature change occurs when temperature is increased or decreased by the flow of heat. [h 2] and [i 2]. Changes in temperature change the equilibrium constant kc. The equilibrium constant shows how. For exothermic and endothermic reactions, this added stress is a change in temperature. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. For an endothermic reaction such as: It does not change if concentrations or pressures. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! Look at the equilibrium involving. This shifts chemical equilibria toward the. K c or k p are constant at constant temperature, but they vary as the temperature changes. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. The equilibrium constant is only affected by temperature.
From www.slideserve.com
PPT Factors that Affect Equilibrium PowerPoint Presentation, free How Does Temperature Change Kc Changes in temperature change the equilibrium constant kc. The equilibrium constant shows how. Look at the equilibrium involving. [h 2] and [i 2]. For exothermic and endothermic reactions, this added stress is a change in temperature. The equilibrium constant is only affected by temperature. But unlike a change in pressure, a change in temperature actually leads to a change in. How Does Temperature Change Kc.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation How Does Temperature Change Kc The value of the equilibrium constant, k, for a given reaction is dependent on temperature. K c or k p are constant at constant temperature, but they vary as the temperature changes. A temperature change occurs when temperature is increased or decreased by the flow of heat. But unlike a change in pressure, a change in temperature actually leads to. How Does Temperature Change Kc.
From slideplayer.com
How does temperature change throughout the ocean? ppt download How Does Temperature Change Kc This shifts chemical equilibria toward the. If δh is positive, reaction is endothermic, then: It does not change if concentrations or pressures. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For an endothermic reaction such as: The equilibrium constant is only affected by temperature. A temperature change occurs when temperature is increased or. How Does Temperature Change Kc.
From tfvp.org
Why Is Kc Affected By Temperature Unveiling The Thermodynamic Mystery How Does Temperature Change Kc The value of the equilibrium constant, k, for a given reaction is dependent on temperature. K c or k p are constant at constant temperature, but they vary as the temperature changes. If δh is positive, reaction is endothermic, then: [h 2] and [i 2]. For an endothermic reaction such as: It does not change if concentrations or pressures. But. How Does Temperature Change Kc.
From tipseri.com
How does temperature change during melting? Tipseri How Does Temperature Change Kc For an endothermic reaction such as: For exothermic and endothermic reactions, this added stress is a change in temperature. This shifts chemical equilibria toward the. It does not change if concentrations or pressures. A temperature change occurs when temperature is increased or decreased by the flow of heat. Since chemists often work at constant temperature and pressure we will follow. How Does Temperature Change Kc.
From learnah.org
3 Changing conditions on Kc and Kp How Does Temperature Change Kc It does not change if concentrations or pressures. A temperature change occurs when temperature is increased or decreased by the flow of heat. For exothermic and endothermic reactions, this added stress is a change in temperature. For an endothermic reaction such as: Look at the equilibrium involving. Equilibrium constants are changed if you change the temperature of the system. [h. How Does Temperature Change Kc.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Does Temperature Change Kc The value of the equilibrium constant, k, for a given reaction is dependent on temperature. It does not change if concentrations or pressures. If δh is positive, reaction is endothermic, then: For exothermic and endothermic reactions, this added stress is a change in temperature. But unlike a change in pressure, a change in temperature actually leads to a change in. How Does Temperature Change Kc.
From slideplayer.com
How does temperature change throughout the ocean? ppt download How Does Temperature Change Kc The equilibrium constant is only affected by temperature. K c or k p are constant at constant temperature, but they vary as the temperature changes. Equilibrium constants are changed if you change the temperature of the system. It does not change if concentrations or pressures. The value of the equilibrium constant, k, for a given reaction is dependent on temperature.. How Does Temperature Change Kc.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching How Does Temperature Change Kc Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. If δh is positive, reaction is endothermic, then: For an endothermic reaction such as: A temperature change occurs when temperature is increased or decreased by the flow of heat. The value of the equilibrium constant, k, for a given reaction. How Does Temperature Change Kc.
From slideplayer.com
Warmup discussion Imagine that you are at the beach, and you get into How Does Temperature Change Kc The equilibrium constant shows how. This shifts chemical equilibria toward the. The equilibrium constant is only affected by temperature. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. Equilibrium constants are changed if you change the temperature of the system. The value of the equilibrium constant, k, for a. How Does Temperature Change Kc.
From www.chegg.com
3. The effect of temperature on Kp is given by And How Does Temperature Change Kc Look at the equilibrium involving. The effect of temperature on kc and kp. If δh is positive, reaction is endothermic, then: Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. For exothermic and endothermic reactions, this added stress is a change in temperature. [h 2] and [i 2]. For. How Does Temperature Change Kc.
From www.electricity-magnetism.org
How does temperature affect resistance? How Does Temperature Change Kc For an endothermic reaction such as: [h 2] and [i 2]. It does not change if concentrations or pressures. The equilibrium constant is only affected by temperature. If δh is positive, reaction is endothermic, then: For exothermic and endothermic reactions, this added stress is a change in temperature. The equilibrium constant shows how. Changes in temperature change the equilibrium constant. How Does Temperature Change Kc.
From www.bethroars.com
You Can Hear Cold — Beth Roars How Does Temperature Change Kc But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! The effect of temperature on kc and kp. The equilibrium constant shows how. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. It does not change if concentrations or pressures. Equilibrium constants. How Does Temperature Change Kc.
From www.electricity-magnetism.org
How does temperature affect electrical conductivity? How Does Temperature Change Kc The effect of temperature on kc and kp. It does not change if concentrations or pressures. Equilibrium constants are changed if you change the temperature of the system. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For exothermic and endothermic reactions, this added stress is a change in temperature. For an endothermic reaction. How Does Temperature Change Kc.
From techiescientist.com
Does Density Change With Temperature? Techiescientist How Does Temperature Change Kc For exothermic and endothermic reactions, this added stress is a change in temperature. It does not change if concentrations or pressures. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. The equilibrium constant shows how. This shifts chemical equilibria toward the. The equilibrium constant is only affected by temperature.. How Does Temperature Change Kc.
From www.kevenoll.com.br
How Sudden Temperature Changes Impact Our Health How Does Temperature Change Kc The effect of temperature on kc and kp. The equilibrium constant is only affected by temperature. If δh is positive, reaction is endothermic, then: For exothermic and endothermic reactions, this added stress is a change in temperature. It does not change if concentrations or pressures. This shifts chemical equilibria toward the. Look at the equilibrium involving. [h 2] and [i. How Does Temperature Change Kc.
From www.chegg.com
How does temperature change the speed of gas How Does Temperature Change Kc The effect of temperature on kc and kp. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. The equilibrium constant shows how. Look at the equilibrium involving. K c or k p are constant at constant temperature, but they vary as the temperature changes. [h 2] and [i 2].. How Does Temperature Change Kc.
From joifcnqsy.blob.core.windows.net
Does Partial Pressure Increase With Temperature at Linda Adams blog How Does Temperature Change Kc Changes in temperature change the equilibrium constant kc. A temperature change occurs when temperature is increased or decreased by the flow of heat. For an endothermic reaction such as: This shifts chemical equilibria toward the. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Equilibrium constants are changed if you change the temperature of. How Does Temperature Change Kc.
From www.cgpplus.co.uk
How Does Temperature Affect Evaporation? (Year 4) CGP Plus How Does Temperature Change Kc Equilibrium constants are changed if you change the temperature of the system. For exothermic and endothermic reactions, this added stress is a change in temperature. The equilibrium constant is only affected by temperature. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. This shifts chemical equilibria toward the. The effect of temperature on kc. How Does Temperature Change Kc.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent How Does Temperature Change Kc K c or k p are constant at constant temperature, but they vary as the temperature changes. Look at the equilibrium involving. If δh is positive, reaction is endothermic, then: The equilibrium constant shows how. It does not change if concentrations or pressures. Changes in temperature change the equilibrium constant kc. Since chemists often work at constant temperature and pressure. How Does Temperature Change Kc.
From www.youtube.com
19 Only temperature affects Kc YouTube How Does Temperature Change Kc The value of the equilibrium constant, k, for a given reaction is dependent on temperature. It does not change if concentrations or pressures. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. A temperature change occurs when temperature is increased or decreased by the flow of heat. [h 2]. How Does Temperature Change Kc.
From slideplayer.com
How does temperature change throughout the ocean? ppt download How Does Temperature Change Kc But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! Look at the equilibrium involving. A temperature change occurs when temperature is increased or decreased by the flow of heat. This shifts chemical equilibria toward the. If δh is positive, reaction is endothermic, then: Changes in temperature change. How Does Temperature Change Kc.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science How Does Temperature Change Kc The effect of temperature on kc and kp. For exothermic and endothermic reactions, this added stress is a change in temperature. The equilibrium constant shows how. If δh is positive, reaction is endothermic, then: But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! The value of the. How Does Temperature Change Kc.
From slideplayer.com
How does temperature change throughout the ocean? ppt download How Does Temperature Change Kc The effect of temperature on kc and kp. Equilibrium constants are changed if you change the temperature of the system. Changes in temperature change the equilibrium constant kc. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. The equilibrium constant is only affected by temperature. A temperature change occurs when temperature is increased or. How Does Temperature Change Kc.
From haipernews.com
How To Calculate Equilibrium Constant Chemistry Haiper How Does Temperature Change Kc For an endothermic reaction such as: For exothermic and endothermic reactions, this added stress is a change in temperature. The equilibrium constant shows how. The equilibrium constant is only affected by temperature. This shifts chemical equilibria toward the. Changes in temperature change the equilibrium constant kc. K c or k p are constant at constant temperature, but they vary as. How Does Temperature Change Kc.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Does Temperature Change Kc For exothermic and endothermic reactions, this added stress is a change in temperature. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Since chemists often work at constant temperature and pressure we. How Does Temperature Change Kc.
From www.slideserve.com
PPT Le Chatelier’s Principle PowerPoint Presentation, free download How Does Temperature Change Kc It does not change if concentrations or pressures. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! Changes in temperature change the equilibrium constant kc. If δh is positive, reaction is endothermic, then: The equilibrium constant shows how. For exothermic and endothermic reactions, this added stress is. How Does Temperature Change Kc.
From xo-b.com
In Which of the Following Reactions Will Kc Kp How Does Temperature Change Kc This shifts chemical equilibria toward the. A temperature change occurs when temperature is increased or decreased by the flow of heat. It does not change if concentrations or pressures. For exothermic and endothermic reactions, this added stress is a change in temperature. Equilibrium constants are changed if you change the temperature of the system. For an endothermic reaction such as:. How Does Temperature Change Kc.
From www.youtube.com
Effect of Temperature on Equilibrium Constants (Extension Material How Does Temperature Change Kc For exothermic and endothermic reactions, this added stress is a change in temperature. If δh is positive, reaction is endothermic, then: But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For an. How Does Temperature Change Kc.
From www.youtube.com
Temperature Change YouTube How Does Temperature Change Kc [h 2] and [i 2]. Changes in temperature change the equilibrium constant kc. Equilibrium constants are changed if you change the temperature of the system. If δh is positive, reaction is endothermic, then: The value of the equilibrium constant, k, for a given reaction is dependent on temperature. For an endothermic reaction such as: Look at the equilibrium involving. It. How Does Temperature Change Kc.
From letstalkscience.ca
Weather Temperature Let's Talk Science How Does Temperature Change Kc Equilibrium constants are changed if you change the temperature of the system. [h 2] and [i 2]. But unlike a change in pressure, a change in temperature actually leads to a change in the value of the equilibrium constant! Look at the equilibrium involving. The equilibrium constant is only affected by temperature. The effect of temperature on kc and kp.. How Does Temperature Change Kc.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws How Does Temperature Change Kc Changes in temperature change the equilibrium constant kc. This shifts chemical equilibria toward the. It does not change if concentrations or pressures. If δh is positive, reaction is endothermic, then: [h 2] and [i 2]. The equilibrium constant is only affected by temperature. K c or k p are constant at constant temperature, but they vary as the temperature changes.. How Does Temperature Change Kc.
From wtffunfact.com
WTF Fun Fact 12978 How Does Temperature Affect the Color of Leaves? How Does Temperature Change Kc The effect of temperature on kc and kp. Look at the equilibrium involving. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. K c or k p are constant at constant temperature, but they vary as the temperature changes. It does not change if concentrations or pressures. The equilibrium constant shows how. A temperature. How Does Temperature Change Kc.
From www.tec-science.com
Why does the temperature remain constant during a change of state How Does Temperature Change Kc Equilibrium constants are changed if you change the temperature of the system. The effect of temperature on kc and kp. For exothermic and endothermic reactions, this added stress is a change in temperature. This shifts chemical equilibria toward the. For an endothermic reaction such as: The value of the equilibrium constant, k, for a given reaction is dependent on temperature.. How Does Temperature Change Kc.
From sciencenotes.org
What Is Temperature? Definition in Science How Does Temperature Change Kc [h 2] and [i 2]. The value of the equilibrium constant, k, for a given reaction is dependent on temperature. Look at the equilibrium involving. The equilibrium constant is only affected by temperature. Since chemists often work at constant temperature and pressure we will follow the spontaneity of this reaction following the changes. But unlike a change in pressure, a. How Does Temperature Change Kc.