Fda Labeling Requirements Medical Devices . Name and place of business of manufacturer, packer or distributor. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; Intended use of the device. (a) the label of a device in. Manufacturer’s name and business location. Name and place of business of.
from mavink.com
§ 801.1 medical devices; food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. Intended use of the device. Manufacturer’s name and business location.
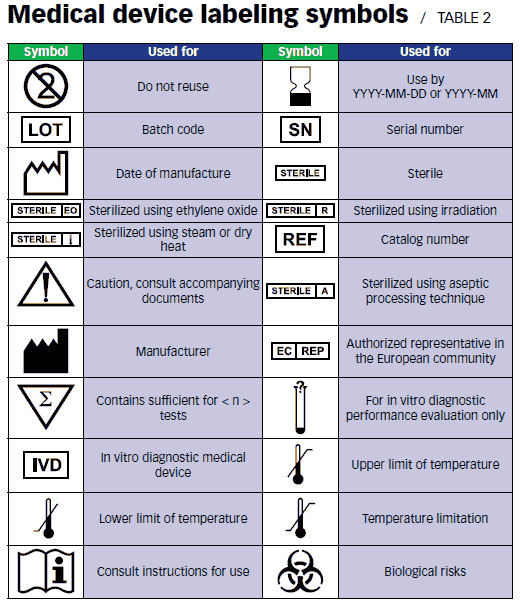
Medical Device Labeling Symbols
Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. (a) the label of a device in. Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; Manufacturer’s name and business location. Name and place of business of. Intended use of the device.
From www.greenlight.guru
FDA Labeling Requirements Checklist Free Download Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Name and place of business of. (a) the label of a device in. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; food and drug administration, department of health and human. Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Overview RegDesk Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Manufacturer’s name and business location. Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. food and drug administration, department of health and human services. Intended use of the device. (a). Fda Labeling Requirements Medical Devices.
From hiveta.com
Label Compliance AB&R® (American Barcode and RFID) Fda Labeling Requirements Medical Devices Name and place of business of. § 801.1 medical devices; Manufacturer’s name and business location. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of manufacturer, packer or distributor. Intended. Fda Labeling Requirements Medical Devices.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Fda Labeling Requirements Medical Devices § 801.1 medical devices; food and drug administration, department of health and human services. Manufacturer’s name and business location. (a) the label of a device in. Name and place of business of manufacturer, packer or distributor. Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of. Fda Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Labeling Requirements Medical Devices Name and place of business of. Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; (a) the label of a device in. Intended use of the device. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21. Fda Labeling Requirements Medical Devices.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements Medical Devices food and drug administration, department of health and human services. Manufacturer’s name and business location. (a) the label of a device in. § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of manufacturer, packer or distributor.. Fda Labeling Requirements Medical Devices.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Labeling Requirements Medical Devices Intended use of the device. (a) the label of a device in. Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; Manufacturer’s name and business location. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of. Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Labeling Requirements Medical Devices food and drug administration, department of health and human services. Manufacturer’s name and business location. (a) the label of a device in. § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the device. Name and place of business. Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on Medical Device Patient Labeling Overview RegDesk Fda Labeling Requirements Medical Devices food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of. Name and place of business of manufacturer, packer or distributor. Intended use of the device. (a) the label of a device. Fda Labeling Requirements Medical Devices.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Labeling Requirements Medical Devices § 801.1 medical devices; Name and place of business of. Manufacturer’s name and business location. Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the device. food and drug administration, department of. Fda Labeling Requirements Medical Devices.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Labeling Requirements Medical Devices food and drug administration, department of health and human services. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. Name and place of business of. Manufacturer’s name and business location. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal. Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Labeling Requirements Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; Name and place of business of. Intended use of the device. food and drug administration, department of health and human services. Manufacturer’s name and business location. Name and place of business of. Fda Labeling Requirements Medical Devices.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Manufacturer’s name and business location. Intended use of the device. § 801.1 medical devices; Name and place of business of. (a) the label of a device in. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations.. Fda Labeling Requirements Medical Devices.
From www.youtube.com
U.S. FDA's Unique Device Identifier (UDI) Requirements YouTube Fda Labeling Requirements Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Manufacturer’s name and business location. food and drug administration, department of health and human services. Name and place of business of. Name and place of business of manufacturer, packer or distributor. (a) the label of a device. Fda Labeling Requirements Medical Devices.
From old.sermitsiaq.ag
Medical Device Label Template Fda Labeling Requirements Medical Devices Name and place of business of. food and drug administration, department of health and human services. § 801.1 medical devices; (a) the label of a device in. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the device. Name and place of. Fda Labeling Requirements Medical Devices.
From www.youtube.com
FDA Requirements for Device Labeling YouTube Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. Name and place of business of. (a) the label of a device in. § 801.1 medical devices; food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of. Fda Labeling Requirements Medical Devices.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements Medical Devices Intended use of the device. Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Manufacturer’s name and business location. Name and place of business of. food and drug administration, department of health and human services. (a). Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Labeling Requirements Medical Devices Intended use of the device. (a) the label of a device in. Manufacturer’s name and business location. Name and place of business of manufacturer, packer or distributor. food and drug administration, department of health and human services. Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21. Fda Labeling Requirements Medical Devices.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Fda Labeling Requirements Medical Devices § 801.1 medical devices; Manufacturer’s name and business location. food and drug administration, department of health and human services. Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. (a) the label of a device in. Name and place of business. Fda Labeling Requirements Medical Devices.
From www.presentationeze.com
FDA Medical Device Labeling.PresentationEZE Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Manufacturer’s name and business location. (a) the label of a device in. food and drug administration, department of health and human services. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the device.. Fda Labeling Requirements Medical Devices.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Manufacturer’s name and business location. § 801.1 medical devices; food and drug administration, department of health and human services. Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and. Fda Labeling Requirements Medical Devices.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Labeling Requirements Medical Devices labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. (a) the label of a device in. Name and place of business of. food and drug administration, department of health and human services. Name and place of business of manufacturer, packer or distributor. § 801.1 medical. Fda Labeling Requirements Medical Devices.
From www.vrogue.co
Fda Medical Device Labeling Regulations Archives Medi vrogue.co Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Intended use of the device. (a) the label of a device in. Manufacturer’s name and business location. food and drug administration, department of health and human services. Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21. Fda Labeling Requirements Medical Devices.
From mavink.com
Medical Device Labeling Symbols Fda Labeling Requirements Medical Devices § 801.1 medical devices; Name and place of business of manufacturer, packer or distributor. food and drug administration, department of health and human services. Intended use of the device. (a) the label of a device in. Manufacturer’s name and business location. Name and place of business of. labeling regulations pertaining to medical devices are found in the. Fda Labeling Requirements Medical Devices.
From mungfali.com
FDA Medical Device Label Symbols Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. food and drug administration, department of health and human services. Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of manufacturer, packer or distributor. (a) the label of a device. Fda Labeling Requirements Medical Devices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. (a) the label of a device in. food and drug administration, department of health and human services.. Fda Labeling Requirements Medical Devices.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. food and drug administration, department of health and human services. Intended use of the device. Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. § 801.1 medical devices; (a). Fda Labeling Requirements Medical Devices.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. (a) the label of a device in. Name and place of business of.. Fda Labeling Requirements Medical Devices.
From medicaldeviceacademy.com
FDA medical device labeling regulations Archives Medical Device Academy Fda Labeling Requirements Medical Devices food and drug administration, department of health and human services. § 801.1 medical devices; Name and place of business of. Name and place of business of manufacturer, packer or distributor. (a) the label of a device in. Intended use of the device. Manufacturer’s name and business location. labeling regulations pertaining to medical devices are found in the. Fda Labeling Requirements Medical Devices.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. food and drug administration, department of health and human services. (a) the label of a device in. Intended use of the device. Name and place of business of manufacturer, packer or distributor.. Fda Labeling Requirements Medical Devices.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Labeling Requirements Medical Devices (a) the label of a device in. Name and place of business of. food and drug administration, department of health and human services. § 801.1 medical devices; Manufacturer’s name and business location. Intended use of the device. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal. Fda Labeling Requirements Medical Devices.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Labeling Requirements Medical Devices Name and place of business of manufacturer, packer or distributor. Name and place of business of. food and drug administration, department of health and human services. § 801.1 medical devices; Intended use of the device. Manufacturer’s name and business location. (a) the label of a device in. labeling regulations pertaining to medical devices are found in the. Fda Labeling Requirements Medical Devices.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Labeling Requirements Medical Devices (a) the label of a device in. food and drug administration, department of health and human services. Name and place of business of manufacturer, packer or distributor. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the device. § 801.1 medical devices;. Fda Labeling Requirements Medical Devices.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. food and drug administration, department of health and human services. (a) the label of a device in. § 801.1 medical devices; Name and place of business of. labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Intended use of the. Fda Labeling Requirements Medical Devices.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements Medical Devices Manufacturer’s name and business location. Name and place of business of manufacturer, packer or distributor. § 801.1 medical devices; labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations. Name and place of business of. food and drug administration, department of health and human services. (a). Fda Labeling Requirements Medical Devices.