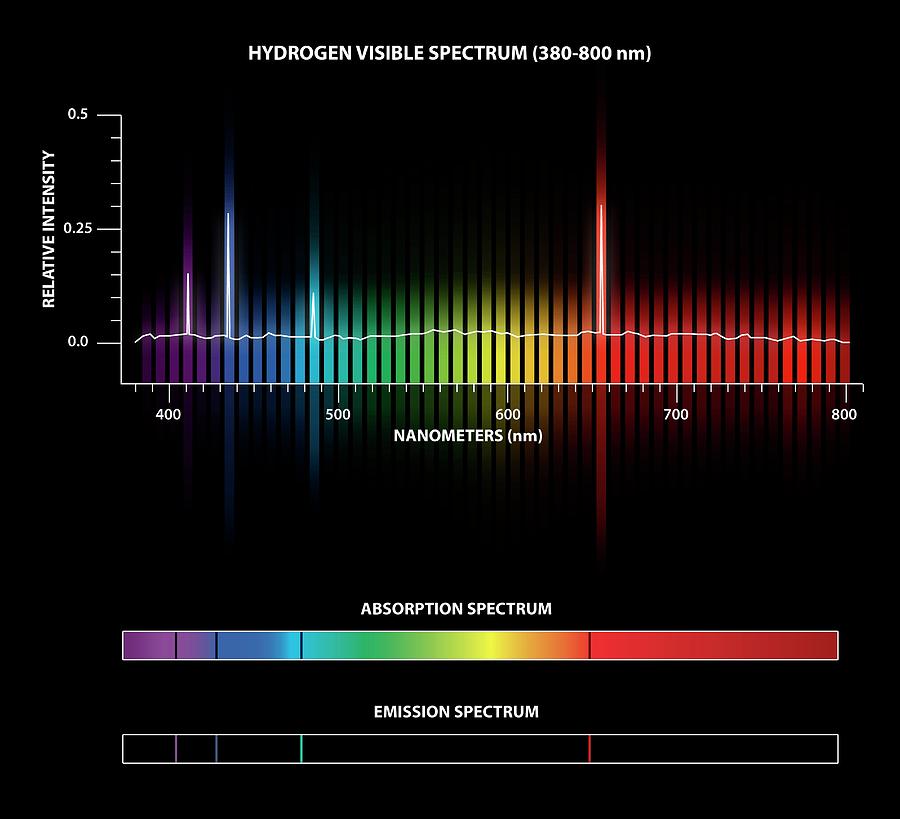
Spectroscopy And Emission Lines . 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. emission spectra lines are the bright lines on the dark background. the pattern of an emission spectrum is the inverse of an absorption spectrum. The easiest approach to selecting a wavelength is. Each emission line has a. Describe what ions are and how they are formed; When white light having all the wavelengths of the. narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. You will be able to distinguish between emission and absorption lines in a spectrum. explain how emission line spectra and absorption line spectra are formed; this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum).
from fineartamerica.com
the pattern of an emission spectrum is the inverse of an absorption spectrum. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). You will be able to distinguish between emission and absorption lines in a spectrum. When white light having all the wavelengths of the. Describe what ions are and how they are formed; The easiest approach to selecting a wavelength is. Each emission line has a. explain how emission line spectra and absorption line spectra are formed; 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector.
Hydrogen Emission And Absorption Spectra Photograph by Carlos Clarivan
Spectroscopy And Emission Lines You will be able to distinguish between emission and absorption lines in a spectrum. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). emission spectra lines are the bright lines on the dark background. You will be able to distinguish between emission and absorption lines in a spectrum. explain how emission line spectra and absorption line spectra are formed; The easiest approach to selecting a wavelength is. Each emission line has a. the pattern of an emission spectrum is the inverse of an absorption spectrum. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. When white light having all the wavelengths of the. Describe what ions are and how they are formed;
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Spectroscopy And Emission Lines explain how emission line spectra and absorption line spectra are formed; You will be able to distinguish between emission and absorption lines in a spectrum. emission spectra lines are the bright lines on the dark background. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in. Spectroscopy And Emission Lines.
From www.diamond.ac.uk
Xray Emission Spectroscopy Diamond Light Source Spectroscopy And Emission Lines You will be able to distinguish between emission and absorption lines in a spectrum. explain how emission line spectra and absorption line spectra are formed; emission spectra lines are the bright lines on the dark background. When white light having all the wavelengths of the. 101 rows a spectral line is a weaker or stronger region in. Spectroscopy And Emission Lines.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Spectroscopy And Emission Lines emission spectra lines are the bright lines on the dark background. You will be able to distinguish between emission and absorption lines in a spectrum. explain how emission line spectra and absorption line spectra are formed; Describe what ions are and how they are formed; 101 rows a spectral line is a weaker or stronger region in. Spectroscopy And Emission Lines.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectroscopy And Emission Lines You will be able to distinguish between emission and absorption lines in a spectrum. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. explain how emission line spectra and absorption line spectra are formed; Describe what ions are and how they are formed; emission spectra lines are the. Spectroscopy And Emission Lines.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Spectroscopy And Emission Lines the pattern of an emission spectrum is the inverse of an absorption spectrum. When white light having all the wavelengths of the. Describe what ions are and how they are formed; Each emission line has a. The easiest approach to selecting a wavelength is. emission spectra lines are the bright lines on the dark background. 101 rows. Spectroscopy And Emission Lines.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy And Emission Lines narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. You will be able to distinguish between emission and absorption lines in a spectrum. Each emission line has a. Describe what ions are and how they are formed; When white light having all the wavelengths of the. 101 rows a spectral line. Spectroscopy And Emission Lines.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Spectroscopy And Emission Lines Describe what ions are and how they are formed; Each emission line has a. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. the pattern of an emission spectrum is the inverse of an absorption spectrum. The easiest approach to selecting a wavelength is. this spectrum consists of. Spectroscopy And Emission Lines.
From pages.uoregon.edu
Astronomy 122 Spectroscopy Spectroscopy And Emission Lines explain how emission line spectra and absorption line spectra are formed; Each emission line has a. The easiest approach to selecting a wavelength is. When white light having all the wavelengths of the. the pattern of an emission spectrum is the inverse of an absorption spectrum. You will be able to distinguish between emission and absorption lines in. Spectroscopy And Emission Lines.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Spectroscopy And Emission Lines narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. explain how emission line spectra and absorption line spectra are formed; the pattern of an emission spectrum is the inverse of an absorption spectrum. Each emission line has a. The easiest approach to selecting a wavelength is. this spectrum consists. Spectroscopy And Emission Lines.
From www.chemistrysources.com
Hydrogen Atomic Spectrum مصادر الكيمياء Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. emission spectra lines are the bright lines on the dark background. You will be able to distinguish between emission and absorption lines in a spectrum. explain how emission line spectra and. Spectroscopy And Emission Lines.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. You will be able to distinguish between emission and absorption lines in a spectrum. emission spectra lines are the bright lines on the dark background. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. this spectrum consists of seven distinct. Spectroscopy And Emission Lines.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Spectroscopy And Emission Lines the pattern of an emission spectrum is the inverse of an absorption spectrum. Each emission line has a. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector.. Spectroscopy And Emission Lines.
From comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Spectroscopy And Emission Lines Describe what ions are and how they are formed; explain how emission line spectra and absorption line spectra are formed; this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). emission spectra lines are the bright lines on the dark background. You will be. Spectroscopy And Emission Lines.
From spiff.rit.edu
Emission and Absorption Lines Spectroscopy And Emission Lines 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. You will be able to distinguish between emission and absorption lines in a spectrum. Describe what ions are and how they are formed; Each emission line has a. narrower slit widths provide better resolution, but at the cost of less. Spectroscopy And Emission Lines.
From fineartamerica.com
Hydrogen Emission And Absorption Spectra Photograph by Carlos Clarivan Spectroscopy And Emission Lines Describe what ions are and how they are formed; the pattern of an emission spectrum is the inverse of an absorption spectrum. emission spectra lines are the bright lines on the dark background. You will be able to distinguish between emission and absorption lines in a spectrum. Each emission line has a. explain how emission line spectra. Spectroscopy And Emission Lines.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy And Emission Lines emission spectra lines are the bright lines on the dark background. The easiest approach to selecting a wavelength is. Each emission line has a. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). narrower slit widths provide better resolution, but at the cost. Spectroscopy And Emission Lines.
From www.resonancescience.org
What is Resonance and Why is it so Important? Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. the pattern of an emission spectrum is the inverse of an absorption spectrum. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Describe what ions are and how they are formed; emission spectra lines are the bright lines on the. Spectroscopy And Emission Lines.
From www.chem1.com
Light, particles and waves Spectroscopy And Emission Lines the pattern of an emission spectrum is the inverse of an absorption spectrum. explain how emission line spectra and absorption line spectra are formed; narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. When white light having all the wavelengths of the. Describe what ions are and how they are. Spectroscopy And Emission Lines.
From www.dreamstime.com
The Spectrum Vector Diagram Stock Vector Illustration Spectroscopy And Emission Lines narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum.. Spectroscopy And Emission Lines.
From byjus.com
Emission spectrum Atomic spectra Chemistry Spectroscopy And Emission Lines When white light having all the wavelengths of the. Describe what ions are and how they are formed; You will be able to distinguish between emission and absorption lines in a spectrum. The easiest approach to selecting a wavelength is. the pattern of an emission spectrum is the inverse of an absorption spectrum. this spectrum consists of seven. Spectroscopy And Emission Lines.
From www.sciencephoto.com
Hydrogen and helium spectra Stock Image C025/0251 Science Photo Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. explain how emission line spectra and absorption line. Spectroscopy And Emission Lines.
From www.astronoo.com
Spectroscopy Spectroscopy And Emission Lines When white light having all the wavelengths of the. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Each emission line has a. Describe what ions are and how they are formed; emission spectra lines are the bright lines on the dark background. narrower slit widths provide better. Spectroscopy And Emission Lines.
From saylordotorg.github.io
Atomic Spectra and Models of the Atom Spectroscopy And Emission Lines 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Describe what ions are and how they are formed; explain how emission line spectra and absorption line spectra are formed; You will be able to distinguish between emission and absorption lines in a spectrum. When white light having all the. Spectroscopy And Emission Lines.
From spiff.rit.edu
Spectrographs and Spectra Spectroscopy And Emission Lines narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. emission spectra lines are the bright lines on the dark background. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Describe what ions are and how they are formed; The easiest approach. Spectroscopy And Emission Lines.
From studyposter.blogspot.com
How Is A Stars Emission Spectrum Used To Study Stars Study Poster Spectroscopy And Emission Lines this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). Describe what ions are and how they are formed; 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. When white light having all the wavelengths of. Spectroscopy And Emission Lines.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Spectroscopy And Emission Lines explain how emission line spectra and absorption line spectra are formed; emission spectra lines are the bright lines on the dark background. narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. The easiest approach to selecting a wavelength is. 101 rows a spectral line is a weaker or stronger. Spectroscopy And Emission Lines.
From jila.colorado.edu
4. Spectroscopy And Emission Lines Each emission line has a. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). explain how emission line spectra and absorption line spectra are formed; emission spectra lines are the bright lines on the dark background. narrower slit widths provide better resolution,. Spectroscopy And Emission Lines.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Spectroscopy And Emission Lines explain how emission line spectra and absorption line spectra are formed; When white light having all the wavelengths of the. Describe what ions are and how they are formed; emission spectra lines are the bright lines on the dark background. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and. Spectroscopy And Emission Lines.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Spectroscopy And Emission Lines When white light having all the wavelengths of the. You will be able to distinguish between emission and absorption lines in a spectrum. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). The easiest approach to selecting a wavelength is. explain how emission line. Spectroscopy And Emission Lines.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). You will be able to distinguish between emission and absorption lines. Spectroscopy And Emission Lines.
From pixels.com
Helium Emission And Absorption Spectra Photograph by Carlos Clarivan Spectroscopy And Emission Lines You will be able to distinguish between emission and absorption lines in a spectrum. When white light having all the wavelengths of the. Each emission line has a. the pattern of an emission spectrum is the inverse of an absorption spectrum. The easiest approach to selecting a wavelength is. explain how emission line spectra and absorption line spectra. Spectroscopy And Emission Lines.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectroscopy And Emission Lines The easiest approach to selecting a wavelength is. explain how emission line spectra and absorption line spectra are formed; this spectrum consists of seven distinct emission lines (the first two differ by only 0.4 nm and are not resolved in this spectrum). Each emission line has a. narrower slit widths provide better resolution, but at the cost. Spectroscopy And Emission Lines.
From learningschoolerneuern4p.z19.web.core.windows.net
The Spectra Of Atoms Reveals Their Spectroscopy And Emission Lines narrower slit widths provide better resolution, but at the cost of less radiation reaching the detector. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. Describe what ions are and how they are formed; You will be able to distinguish between emission and absorption lines in a spectrum. . Spectroscopy And Emission Lines.
From www.researchgate.net
The atmospheric emission spectrum showing the carbon dioxide band at 4 Spectroscopy And Emission Lines Each emission line has a. explain how emission line spectra and absorption line spectra are formed; 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. the pattern of an emission spectrum is the inverse of an absorption spectrum. emission spectra lines are the bright lines on the. Spectroscopy And Emission Lines.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Spectroscopy And Emission Lines When white light having all the wavelengths of the. Each emission line has a. 101 rows a spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. explain how emission line spectra and absorption line spectra are formed; the pattern of an emission spectrum is the inverse of an absorption spectrum. The. Spectroscopy And Emission Lines.