What Is Electrode Reaction . electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. In an electrochemical cell, an. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. — the electrode provides a surface for oxidation reduction reactions in the cells. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an.
from www.youtube.com
In an electrochemical cell, an. — the electrode provides a surface for oxidation reduction reactions in the cells. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer.
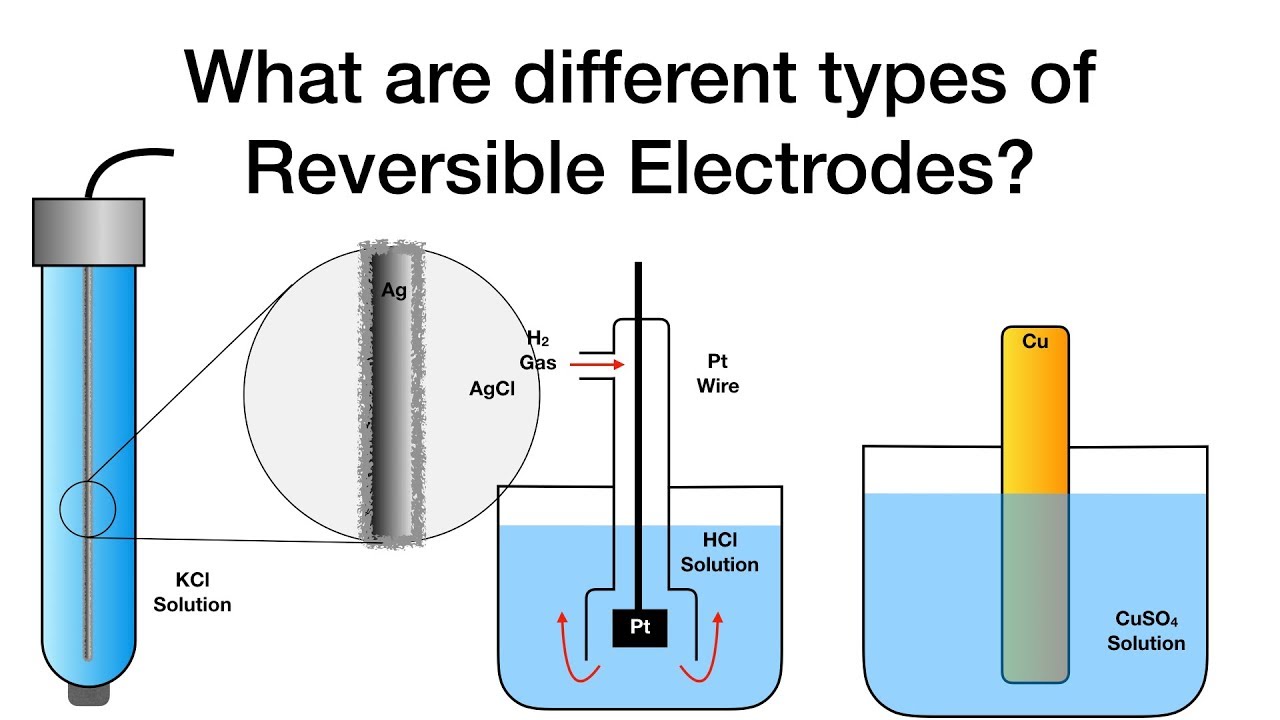
What are different types of Reversible Electrodes? Electrochemistry
What Is Electrode Reaction In an electrochemical cell, an. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. In an electrochemical cell, an.
From www.researchgate.net
2. Pathway of a general electrode reaction and processes in an What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. — electrode reactions are a. What Is Electrode Reaction.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry What Is Electrode Reaction In an electrochemical cell, an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. — the electrode provides a surface for oxidation reduction reactions in the cells. an electrode reaction. What Is Electrode Reaction.
From www.researchgate.net
Pathway of a general electrode reaction. Download Scientific Diagram What Is Electrode Reaction — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. — the electrode provides a surface for oxidation reduction reactions in the cells. an electrode reaction. What Is Electrode Reaction.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. the. What Is Electrode Reaction.
From usermanualtractors.z1.web.core.windows.net
What Happens At The Cathode In Electrolysis What Is Electrode Reaction the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving. What Is Electrode Reaction.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. In an electrochemical cell, an. — the electrode provides a surface for oxidation reduction reactions in the cells. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species. What Is Electrode Reaction.
From www.slideserve.com
PPT Chapter 3 of Electrode Reactions PowerPoint Presentation What Is Electrode Reaction In an electrochemical cell, an. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. — the electrode provides a surface for oxidation reduction reactions in the cells. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across. What Is Electrode Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. . What Is Electrode Reaction.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Is Electrode Reaction the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. — the electrode provides a surface for oxidation reduction reactions in the cells. In an electrochemical cell, an. . What Is Electrode Reaction.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts What Is Electrode Reaction the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. In an electrochemical cell, an. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. — electrode reactions are a class of chemical reactions. What Is Electrode Reaction.
From www.snexplores.org
Explainer What is an electrode? What Is Electrode Reaction — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. In an electrochemical cell, an. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode.. What Is Electrode Reaction.
From www.researchgate.net
1 Pathway of a general electrode reaction. Download Scientific Diagram What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. In an electrochemical cell, an. an electrode reaction refers to the net oxidation or reduction process that takes place. What Is Electrode Reaction.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. — the electrode provides a surface for oxidation reduction reactions in the cells. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. In an electrochemical. What Is Electrode Reaction.
From chemistry.stackexchange.com
electrochemistry Understanding the concept of voltage in electrode What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. In an electrochemical cell, an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. an electrode reaction refers to the net oxidation or reduction process. What Is Electrode Reaction.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. —. What Is Electrode Reaction.
From byjus.com
What is an active electrode? Explain with the help of an example What Is Electrode Reaction the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most. What Is Electrode Reaction.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic What Is Electrode Reaction In an electrochemical cell, an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — the electrode provides a surface for oxidation reduction reactions in the cells. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. an electrode reaction. What Is Electrode Reaction.
From www.youtube.com
REDOX REACTIONS AND ELECTRODE PROCESSES YouTube What Is Electrode Reaction — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode.. What Is Electrode Reaction.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic What Is Electrode Reaction In an electrochemical cell, an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — the electrode provides a surface for oxidation reduction reactions in the cells. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without. What Is Electrode Reaction.
From www.researchgate.net
What reaction occurs at the platinum counter electrode in nonaqueous 3 What Is Electrode Reaction In an electrochemical cell, an. — the electrode provides a surface for oxidation reduction reactions in the cells. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. an. What Is Electrode Reaction.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube What Is Electrode Reaction an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In an electrochemical cell, an. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. electrode reaction, the conduction of electrons and ions and the diffusion of reaction. What Is Electrode Reaction.
From www.researchgate.net
Schematic diagram of the electrode reactions for the electrodes with What Is Electrode Reaction an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In an electrochemical cell, an. — the electrode provides a surface for oxidation reduction reactions in the cells. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. electrode reaction,. What Is Electrode Reaction.
From semcouniversity.com
How the three electrode system works Semco University Semco What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across. What Is Electrode Reaction.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download What Is Electrode Reaction In an electrochemical cell, an. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer.. What Is Electrode Reaction.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. — the electrode provides a surface for oxidation reduction reactions in the cells. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. electrochemical reaction, any process either caused or accompanied. What Is Electrode Reaction.
From www.researchgate.net
Schematic representation of a UE cell showing the electrode reactions What Is Electrode Reaction an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In an electrochemical cell, an. — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer.. What Is Electrode Reaction.
From manualpartwedlock88.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram What Is Electrode Reaction In an electrochemical cell, an. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — the electrode provides a surface for oxidation reduction reactions in. What Is Electrode Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrode Reaction electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. In an electrochemical cell, an. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. the function of the electrodes is to bring about reaction between the reactant (fuel. What Is Electrode Reaction.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is Electrode Reaction an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. the. What Is Electrode Reaction.
From www.researchgate.net
What reaction occurs at the platinum counter electrode in nonaqueous 3 What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. In an electrochemical cell, an. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. the function of the electrodes is to bring about reaction between the reactant (fuel. What Is Electrode Reaction.
From chem.libretexts.org
20.2 Standard Electrode Potentials Chemistry LibreTexts What Is Electrode Reaction — the electrode provides a surface for oxidation reduction reactions in the cells. electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. In an electrochemical cell, an. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. . What Is Electrode Reaction.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. — the electrode provides a surface for oxidation reduction reactions in the cells. In an electrochemical cell, an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species. What Is Electrode Reaction.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent What Is Electrode Reaction the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. In an electrochemical cell, an. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. — electrode reactions are a class of chemical reactions that involve the transfer of a. What Is Electrode Reaction.
From www.researchgate.net
Pathway of a general electrode reaction (see [27]). Download What Is Electrode Reaction — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. the function of the electrodes is to bring about reaction between the reactant (fuel or oxygen) and the electrolyte, without itself. electrode reaction, the conduction of electrons and ions and the diffusion of reaction gases are progressing. . What Is Electrode Reaction.
From www.chemistry-teaching-resources.com
chemistry picture What Is Electrode Reaction electrochemical reaction, any process either caused or accompanied by the passage of an electric current and involving in most cases the transfer. an electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. In an electrochemical cell, an. the function of the electrodes is to bring about reaction between the reactant. What Is Electrode Reaction.