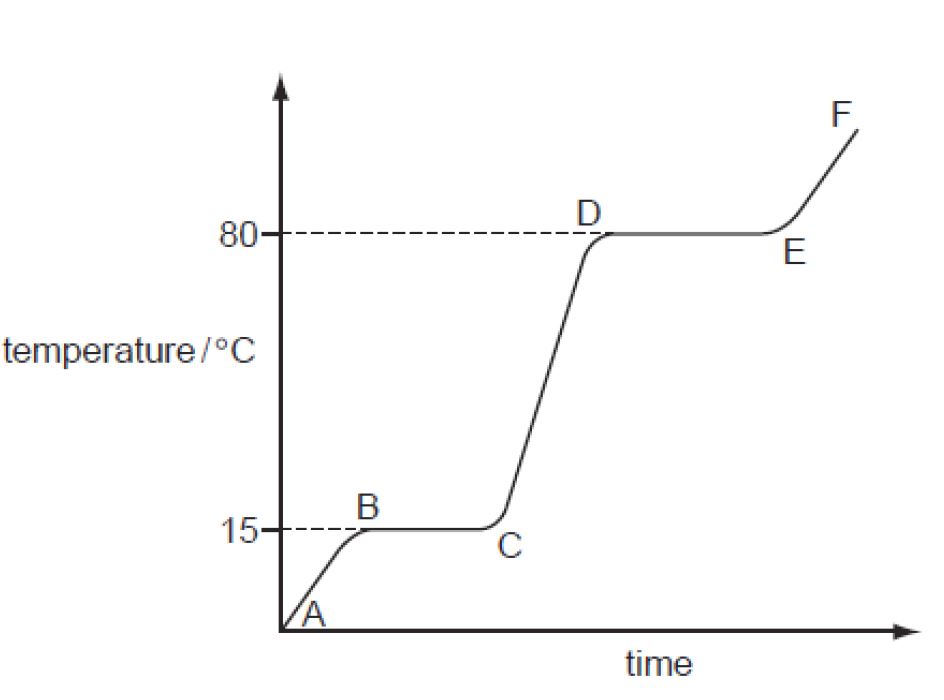
Heating And Cooling Curve Of A Substance Ppt . Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the amount of energy gained or lost by a system be calculated? Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. A heating curve is a plot of the temperature of substance versus the heat added to the. Cooling curves are the opposite and. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. A heating curve is a plot of the temperature of substance versus the heat added to the substance. The graph shows the response of the different phases to added heat. Heating and cooling curves topic:
from www.vrogue.co
A heating curve is a plot of the temperature of substance versus the heat added to the substance. Cooling curves are the opposite and. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. How can the amount of energy gained or lost by a system be calculated? The graph shows the response of the different phases to added heat. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. Heating and cooling curves topic: A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to.
Heating And Cooling Curve Introduction Plus A vrogue.co
Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added to the substance. How can the amount of energy gained or lost by a system be calculated? Heating and cooling curves topic: The graph shows the response of the different phases to added heat. A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Cooling curves are the opposite and. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating And Cooling Curve Of A Substance Ppt The graph shows the response of the different phases to added heat. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added to the substance. A heating/cooling curve is a simple line graph that shows. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Regents Chemistry PowerPoint Presentation, free download ID3170924 Heating And Cooling Curve Of A Substance Ppt Cooling curves are the opposite and. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the amount of energy gained or lost by a system be calculated? A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or. Heating And Cooling Curve Of A Substance Ppt.
From slideplayer.com
Heating and Cooling Curves ppt download Heating And Cooling Curve Of A Substance Ppt The graph shows the response of the different phases to added heat. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. How can the amount of energy gained or lost by a system be calculated? A heating curve is a plot of the temperature of substance versus the. Heating And Cooling Curve Of A Substance Ppt.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating And Cooling Curve Of A Substance Ppt A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating/cooling curve is. Heating And Cooling Curve Of A Substance Ppt.
From slideplayer.com
Thermochemistry. ppt download Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? The graph shows the response of the different phases to added heat. Cooling curves are the opposite and. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Heating and cooling curves topic: Heating curves. Heating And Cooling Curve Of A Substance Ppt.
From www.scribd.com
Heating and Cooling Curve of A Substance PDF Heating And Cooling Curve Of A Substance Ppt Cooling curves are the opposite and. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. The graph shows the response of the different phases to added heat. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating And Cooling Curve Of A Substance Ppt Heating and cooling curves topic: How can the amount of energy gained or lost by a system be calculated? Cooling curves are the opposite and. The graph shows the response of the different phases to added heat. A heating curve is a plot of the temperature of substance versus the heat added to the substance. The curve shows flat regions. Heating And Cooling Curve Of A Substance Ppt.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. Heating and cooling curves topic: The graph shows the response of the different phases to added heat. A heating curve is. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT States of Matter! PowerPoint Presentation, free download ID6851506 Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. How can the amount of energy gained or lost by a system be calculated? The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. Heating curves show flat regions where temperature. Heating And Cooling Curve Of A Substance Ppt.
From coolingchiwayake.blogspot.com
Cooling The Cooling Curve Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the amount of energy gained or lost by a system be calculated? Heating and cooling curves topic: The graph shows the response of the different phases to added heat. A heating curve is a plot of the temperature. Heating And Cooling Curve Of A Substance Ppt.
From slideplayer.com
States of Matter & Energy ppt download Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. A heating/cooling curve is. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? Cooling curves are the opposite and. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Heating and cooling curves topic: Heating curves show flat regions where temperature remains constant as a substance changes phase. Heating And Cooling Curve Of A Substance Ppt.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. A heating curve is a. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Chapter 11. States of Matter PowerPoint Presentation, free Heating And Cooling Curve Of A Substance Ppt A heating curve is a plot of the temperature of substance versus the heat added to the. How can the amount of energy gained or lost by a system be calculated? A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Heating and cooling curves topic: A heating curve. Heating And Cooling Curve Of A Substance Ppt.
From www.scribd.com
Heating and Cooling Curve of A Substance PDF Melting Point Heat Heating And Cooling Curve Of A Substance Ppt The graph shows the response of the different phases to added heat. Cooling curves are the opposite and. Heating and cooling curves topic: How can the amount of energy gained or lost by a system be calculated? A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. A heating. Heating And Cooling Curve Of A Substance Ppt.
From mavink.com
Stearic Acid Cooling Curve Heating And Cooling Curve Of A Substance Ppt The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the. Heating And Cooling Curve Of A Substance Ppt.
From ar.inspiredpencil.com
A Heating Curve Illustrates Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added to the. A heating curve is a plot of the temperature. Heating And Cooling Curve Of A Substance Ppt.
From lessonschoolimbrowning.z14.web.core.windows.net
Heat And Cooling Curve Heating And Cooling Curve Of A Substance Ppt The graph shows the response of the different phases to added heat. A heating curve is a plot of the temperature of substance versus the heat added to the substance. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. How can the amount of energy gained or lost. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Freezing and Boiling Point Graph aka Phase Change Diagram or Heating And Cooling Curve Of A Substance Ppt Heating and cooling curves topic: Cooling curves are the opposite and. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. The graph shows the response of the different phases to added heat. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid. Heating And Cooling Curve Of A Substance Ppt.
From lessonschoolwinchell.z21.web.core.windows.net
Heat And Cooling Curve Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. The graph shows the response of the different phases to added heat. Cooling curves are the opposite and. A heating curve is a plot of the temperature of substance versus the heat added to the substance. The curve shows flat. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Unit 13Thermochemistry PowerPoint Presentation ID5119399 Heating And Cooling Curve Of A Substance Ppt A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added to the substance. The. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT A.P. Chemistry PowerPoint Presentation, free download ID1875178 Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. A heating curve is a plot of the temperature of substance versus the heat added to the substance. The graph shows the response of the different. Heating And Cooling Curve Of A Substance Ppt.
From www.scribd.com
Heating and Cooling Curve of A Substance PDF Phase (Matter Heating And Cooling Curve Of A Substance Ppt A heating curve is a plot of the temperature of substance versus the heat added to the. A heating curve is a plot of the temperature of substance versus the heat added to the substance. How can the amount of energy gained or lost by a system be calculated? The curve shows flat regions where temperature remains constant as heat. Heating And Cooling Curve Of A Substance Ppt.
From studylib.net
Heating and Cooling Curves Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. The graph shows the response of the different phases to added heat. How can the amount of energy gained or lost by a system be calculated? A heating curve is a plot of the temperature of substance versus the heat. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Heating/Cooling Curve and Phase Diagrams PowerPoint Presentation Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the amount of energy gained or lost by a system be calculated? A heating curve is a plot of the temperature of substance versus the heat added to the. Heating curves show flat regions where temperature remains constant. Heating And Cooling Curve Of A Substance Ppt.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating And Cooling Curve Of A Substance Ppt How can the amount of energy gained or lost by a system be calculated? Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. Heating and cooling curves topic: A heating curve is a plot of the temperature of substance versus the heat added to the substance. The graph shows. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. How can the amount of energy gained or lost by a system be calculated? The graph shows the response of the. Heating And Cooling Curve Of A Substance Ppt.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to. Cooling curves are the opposite and. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating curve is a plot of the temperature of substance versus the heat added. Heating And Cooling Curve Of A Substance Ppt.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. The graph shows the response of the different phases to added heat. A heating curve is a plot of the temperature of substance versus the heat added to the substance. A heating curve is a plot of the temperature of. Heating And Cooling Curve Of A Substance Ppt.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heating And Cooling Curve Of A Substance Ppt A heating curve is a plot of the temperature of substance versus the heat added to the. Heating and cooling curves topic: A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. Cooling curves are the opposite and. How can the amount of energy gained or lost by a. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating And Cooling Curve Of A Substance Ppt Heating and cooling curves topic: The graph shows the response of the different phases to added heat. Cooling curves are the opposite and. How can the amount of energy gained or lost by a system be calculated? A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. A heating. Heating And Cooling Curve Of A Substance Ppt.
From slideplayer.com
COOLING CURVE OF PALMITIC ACID LAB ppt download Heating And Cooling Curve Of A Substance Ppt Heating and cooling curves topic: Cooling curves are the opposite and. A heating/cooling curve is a simple line graph that shows the phase changes a given substance undergoes with increasing or decreasing. The graph shows the response of the different phases to added heat. Heating curves show flat regions where temperature remains constant as a substance changes phase from solid. Heating And Cooling Curve Of A Substance Ppt.
From www.vrogue.co
Heating And Cooling Curve Introduction Plus A vrogue.co Heating And Cooling Curve Of A Substance Ppt A heating curve is a plot of the temperature of substance versus the heat added to the substance. Cooling curves are the opposite and. How can the amount of energy gained or lost by a system be calculated? Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. A heating. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Thermodynamics Heating/Cooling Curves PowerPoint Presentation Heating And Cooling Curve Of A Substance Ppt Cooling curves are the opposite and. How can the amount of energy gained or lost by a system be calculated? Heating and cooling curves topic: The graph shows the response of the different phases to added heat. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. Heating curves show. Heating And Cooling Curve Of A Substance Ppt.
From www.slideserve.com
PPT Thermal Properties of Matter (Part I) PowerPoint Presentation Heating And Cooling Curve Of A Substance Ppt Heating curves show flat regions where temperature remains constant as a substance changes phase from solid to liquid to gas. The curve shows flat regions where temperature remains constant as heat is used for phase transitions like melting or vaporization,. The graph shows the response of the different phases to added heat. Heating and cooling curves topic: A heating curve. Heating And Cooling Curve Of A Substance Ppt.