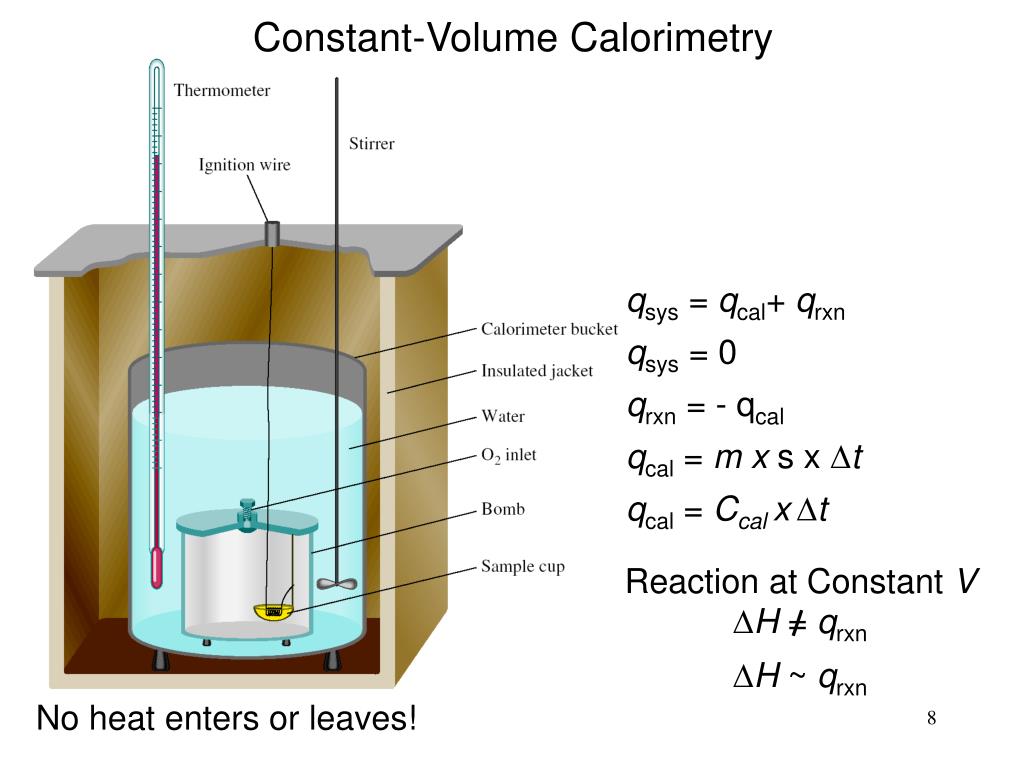
What Is Calorimeter Specific Heat . The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry requires that the material being heated have known thermal properties, i.e. The classical rule, recognized by clausius and by kelvin, is that. It is possible to tell whether a reaction is exothermic (releases heat). It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In the si system, the. 💡 this calculator works in various. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Apply the first law of thermodynamics to calorimetry.
from www.slideserve.com
It is possible to tell whether a reaction is exothermic (releases heat). The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The classical rule, recognized by clausius and by kelvin, is that. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry requires that the material being heated have known thermal properties, i.e. In the si system, the. Apply the first law of thermodynamics to calorimetry. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat.
PPT Calorimetry PowerPoint Presentation, free download ID1875569
What Is Calorimeter Specific Heat When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. 💡 this calculator works in various. The classical rule, recognized by clausius and by kelvin, is that. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Calorimetry requires that the material being heated have known thermal properties, i.e. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It is possible to tell whether a reaction is exothermic (releases heat). The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Apply the first law of thermodynamics to calorimetry. In the si system, the.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimeter Specific Heat It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. The classical rule, recognized by clausius and by kelvin, is that. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1.. What Is Calorimeter Specific Heat.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Calorimeter Specific Heat Apply the first law of thermodynamics to calorimetry. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The classical rule, recognized by clausius and by kelvin, is that. In the si system, the. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat In the si system, the. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Specific heat is the amount of thermal energy you need. What Is Calorimeter Specific Heat.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Is Calorimeter Specific Heat Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Calorimetry requires that the material being heated have known thermal properties, i.e. When. What Is Calorimeter Specific Heat.
From studylib.net
Heat Capacity of Metals PreLab What Is Calorimeter Specific Heat The classical rule, recognized by clausius and by kelvin, is that. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It is. What Is Calorimeter Specific Heat.
From saylordotorg.github.io
Calorimetry What Is Calorimeter Specific Heat Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. Apply the first law of thermodynamics to calorimetry. 💡 this calculator works in various. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. When. What Is Calorimeter Specific Heat.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube What Is Calorimeter Specific Heat The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. In the si system, the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry requires that the. What Is Calorimeter Specific Heat.
From slideplayer.com
Specific Heat. ppt download What Is Calorimeter Specific Heat Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry requires that the material being heated have known thermal properties, i.e. The classical rule, recognized by clausius and by kelvin, is that. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. It. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat Calorimetry requires that the material being heated have known thermal properties, i.e. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat capacity (\(c\)) of a substance,. What Is Calorimeter Specific Heat.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Calorimeter Specific Heat Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The classical rule, recognized by clausius and by kelvin, is that. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat. What Is Calorimeter Specific Heat.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure What Is Calorimeter Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. 💡 this calculator works in various. Compare heat flow from hot to cold objects. What Is Calorimeter Specific Heat.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is Calorimeter Specific Heat When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The specific heat capacity (\(c\)) of a. What Is Calorimeter Specific Heat.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Is Calorimeter Specific Heat 💡 this calculator works in various. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. Calorimetry requires that the material being heated have known thermal properties, i.e. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of. What Is Calorimeter Specific Heat.
From pressbooks.online.ucf.edu
1.4 Heat Transfer, Specific Heat, and Calorimetry General Physics What Is Calorimeter Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry requires that the material being heated have known thermal properties, i.e. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Specific heat is the amount of thermal energy you need. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. Calorimetry requires that the material being heated have known thermal properties, i.e. Apply the first law of thermodynamics to calorimetry. The classical rule, recognized by clausius and by kelvin, is that. Specific heat is the amount of thermal energy you need to. What Is Calorimeter Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo What Is Calorimeter Specific Heat The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. The symbol c stands. What Is Calorimeter Specific Heat.
From users.highland.edu
Calorimetry What Is Calorimeter Specific Heat Apply the first law of thermodynamics to calorimetry. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. It is possible to tell. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat The classical rule, recognized by clausius and by kelvin, is that. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. Specific heat is the amount of thermal energy you need to. What Is Calorimeter Specific Heat.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube What Is Calorimeter Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. 💡 this calculator works in various. Apply the first law of thermodynamics to calorimetry. The classical rule, recognized by clausius and by. What Is Calorimeter Specific Heat.
From www.slideshare.net
Lesson Enthalpy and Calorimetry What Is Calorimeter Specific Heat The classical rule, recognized by clausius and by kelvin, is that. 💡 this calculator works in various. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. In the si system, the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the. What Is Calorimeter Specific Heat.
From www.youtube.com
Calorimetry Calculate Specific Heat YouTube What Is Calorimeter Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. It is possible to tell whether. What Is Calorimeter Specific Heat.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimeter Specific Heat Calorimetry requires that the material being heated have known thermal properties, i.e. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature. What Is Calorimeter Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Is Calorimeter Specific Heat 💡 this calculator works in various. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. In the si system, the. The classical rule, recognized by clausius. What Is Calorimeter Specific Heat.
From chemistrytalk.org
Calorimetry ChemTalk What Is Calorimeter Specific Heat It is possible to tell whether a reaction is exothermic (releases heat). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. Calorimetry requires that the material being heated have known thermal properties, i.e.. What Is Calorimeter Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 What Is Calorimeter Specific Heat In the si system, the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. Compare heat flow from hot to cold objects. What Is Calorimeter Specific Heat.
From studylib.net
Calorimetry Lab Specific Heat Capacity What Is Calorimeter Specific Heat Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. In the si system, the. Apply the first law of thermodynamics to calorimetry. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. The symbol c stands for the. What Is Calorimeter Specific Heat.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards What Is Calorimeter Specific Heat Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Calculate heat, temperature change,. What Is Calorimeter Specific Heat.
From slideplayer.com
Thermal Energy and Heat ppt download What Is Calorimeter Specific Heat Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. It is the technique of calculating how much heat is produced or absorbed while a chemical. What Is Calorimeter Specific Heat.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero What Is Calorimeter Specific Heat Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The classical rule, recognized by clausius and by kelvin, is that. 💡 this calculator works in various. In the si system, the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Specific heat is the. What Is Calorimeter Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is Calorimeter Specific Heat Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. Apply the first law of thermodynamics to calorimetry. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat.. What Is Calorimeter Specific Heat.
From www.youtube.com
Measurement and effects of heat (part 3)! Class 8 Specific heat What Is Calorimeter Specific Heat The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Apply the first law of thermodynamics to calorimetry. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its. What Is Calorimeter Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 What Is Calorimeter Specific Heat 💡 this calculator works in various. Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 k. In the si system, the. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in. What Is Calorimeter Specific Heat.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Calorimeter Specific Heat The classical rule, recognized by clausius and by kelvin, is that. It is possible to tell whether a reaction is exothermic (releases heat). It is the technique of calculating how much heat is produced or absorbed while a chemical reaction occurs. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and. What Is Calorimeter Specific Heat.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Is Calorimeter Specific Heat The classical rule, recognized by clausius and by kelvin, is that. When analysing a heat transfer reaction, chemists use the calorimetry equation relating heat released in the reaction to the substance’s mass, change in temperature, and specific heat. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Apply the. What Is Calorimeter Specific Heat.