What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water . Adding sugar to water increases the boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Indicate what happens to the boiling. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Explain what the term colligative means, and list the colligative properties. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen.
from www.chegg.com
Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Explain what the term colligative means, and list the colligative properties. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Indicate what happens to the boiling. Adding sugar to water increases the boiling point. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at
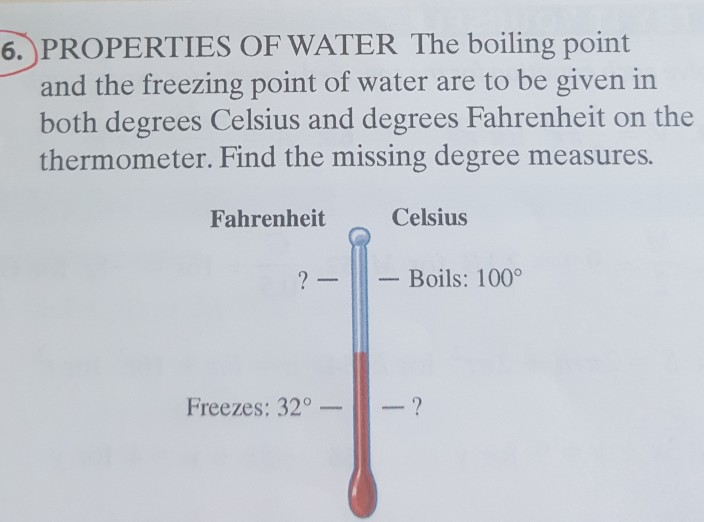
Solved 6. PROPERTIES OF WATER The boiling point and the
What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Indicate what happens to the boiling. Adding sugar to water will raise the boiling point and decrease the freezing point of water. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Explain what the term colligative means, and list the colligative properties. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen.
From www.thoughtco.com
What Is the Freezing Point of Water? What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Indicate what happens to the boiling. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding 1 gram of sugar, or any other substance that. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding sugar to water increases the boiling point. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Explain what the term colligative means, and list the colligative properties. Step by step video & image. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.alamy.com
Freezing melting and boiling point experiment illustration Stock Vector What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.vectorstock.com
Boiling and evaporation freezing and melting Vector Image What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water will raise the boiling point and decrease the freezing point of water. Explain what. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding sugar to water increases the boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From escoffierathome.com
Boiling water and sugar Escoffier At Home What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Explain what the term colligative means, and list the colligative properties. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Indicate what happens to the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.ck12.org
Boiling Point Elevation CK12 Foundation What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Explain what. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From courses.lumenlearning.com
Colligative Properties Chemistry for Majors What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Indicate what happens to the boiling. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Step by step video & image solution for what is the effect of the addition of sugar on the boiling. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water increases the boiling point. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Adding 1 gram of sugar, or any other substance that does not create ions, increases. If 342g of cane sugar is dissolved in 1000 ml of water,. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.toppr.com
Calculate the freezing point and the boiling point at 1 atmosphere of a What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Explain what the term colligative means, and list the colligative properties. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. If 342g of cane sugar is dissolved in 1000 ml of water, the solution. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From vanessa-loksellers.blogspot.com
VanessaLokSellers What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Explain what the term colligative means, and list the colligative properties. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Adding sugar to water will raise the boiling point and decrease. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.chegg.com
Solved 6. PROPERTIES OF WATER The boiling point and the What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Explain what the term colligative means, and list the colligative properties. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water will raise the boiling point and decrease the freezing point of water. Indicate what happens to the boiling. Adding 1 gram of sugar, or any other substance that. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water increases the boiling point.. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.youtube.com
A 5 solution (by mass) of cane sugar in water has freezing point of What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Explain what the term colligative means, and list the colligative properties. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideshare.net
Sugar Boiling in Sugar Crystallization Process What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water increases the boiling point. Indicate what happens to the boiling. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water lowers the freezing point because the sugar molecules prevent the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Indicate what happens to the boiling. Explain what the term colligative means, and list the colligative properties. Step by step video & image solution for what. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.scribd.com
effect on table salt vs table sugar on the boiling point water Sodium What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Indicate what happens to the boiling. Adding sugar to water increases the boiling point. If 342g of cane sugar is dissolved in. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT All that sugar PowerPoint Presentation, free download ID2509901 What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Indicate what happens to the boiling. Explain what the term colligative means, and list the colligative properties. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding sugar to water lowers the freezing point. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Indicate what happens to the boiling. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water lowers the freezing point because the sugar molecules prevent the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From ar.inspiredpencil.com
Boiling Point Elevation Equation What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water increases the boiling point. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Explain what the term colligative means, and list the colligative properties. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. If 342g of. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.ck12.org
Boiling Point Elevation CK12 Foundation What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Adding sugar to water will raise the boiling point and decrease the freezing point of water. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water?. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the boiling point. Explain what the term colligative means, and list the colligative properties. Step by step video & image solution for what. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Indicate what happens to the boiling. Explain what the term colligative means, and list the colligative properties. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water increases the boiling point. Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Indicate what happens to the boiling. Explain what the term colligative means, and list the colligative properties. Adding 1 gram of sugar, or any. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.vrogue.co
Boiling Point Elevation Detailed Explanation With Exa vrogue.co What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Explain what the term colligative means, and list the colligative properties. Indicate what happens to the boiling. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water increases the boiling point. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Adding 1 gram of sugar, or any other substance that. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.chegg.com
Solved 3. Determination of boiling point of Sugar solution What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water?. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Step by step video &. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.gettyimages.in
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Indicate what happens to the boiling. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Explain what the term colligative means, and list the colligative properties. Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Adding sugar to water will raise the boiling point and decrease the freezing point of water. Adding sugar to water lowers the freezing point. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From mavink.com
Elevation In Boiling Point Graph What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making the hydrogen. Regarding boiling point, the boiling point increase (and the freezing point depression) depends only on the number of particles. Indicate what happens to the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Explain what the term colligative means, and list the colligative properties. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding 1 gram of sugar, or any other substance that does not create ions, increases. Indicate what happens to the boiling. Adding sugar to water lowers the freezing point because the sugar. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.engineeringtoolbox.com
Sugar Solubility in Water What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Indicate what happens to the boiling. Adding sugar to water will raise the boiling point and decrease the freezing point of water. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Adding 1 gram of sugar, or any other substance that does not create ions, increases. Adding sugar to water increases the. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water Step by step video & image solution for what is the effect of the addition of sugar on the boiling and freezing points of water? Explain what the term colligative means, and list the colligative properties. If 342g of cane sugar is dissolved in 1000 ml of water, the solution will freeze at Regarding boiling point, the boiling point increase. What Is The Effect Of Addition Of Sugar On The Boiling And Freezing Point Of Water.