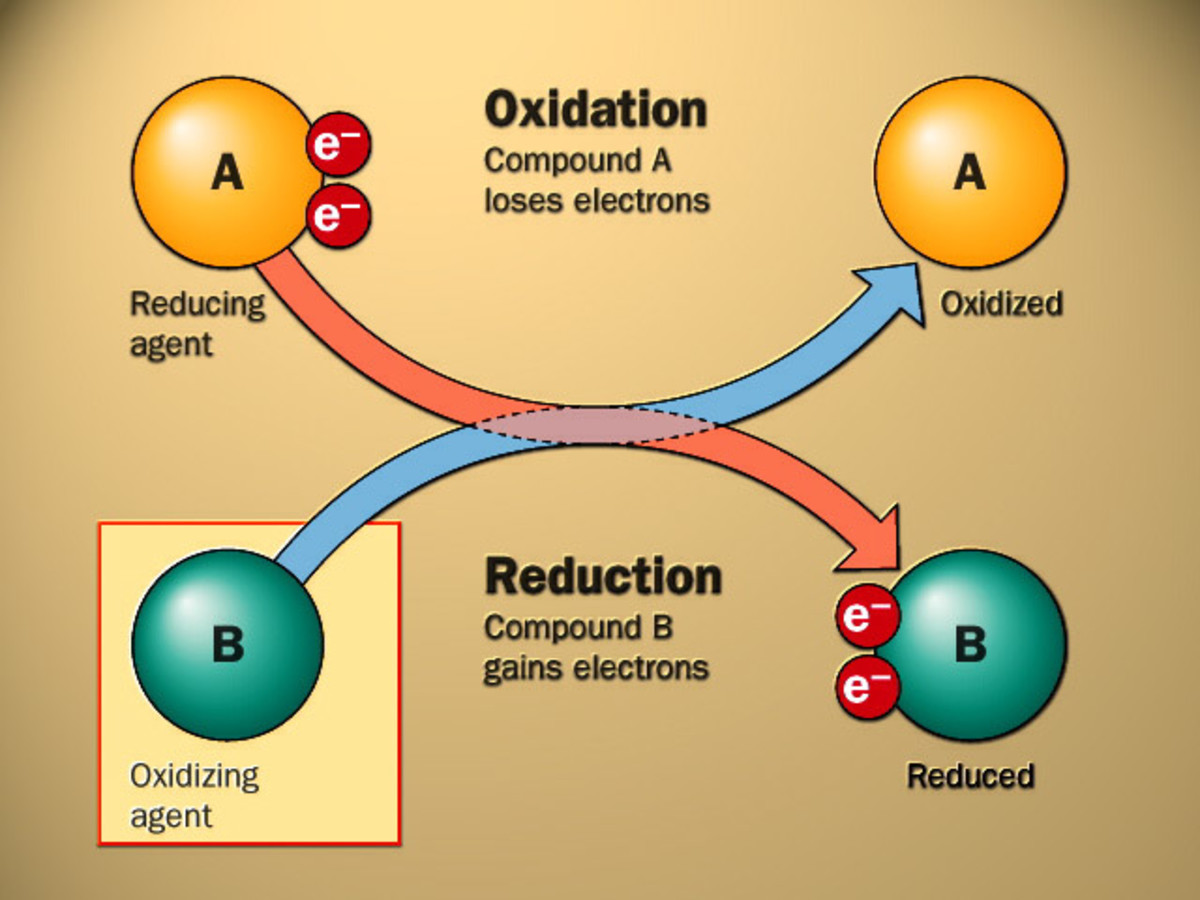
Iron Oxidation Energy . Iron is very easily oxidized under alkaline conditions. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of.
from owlcation.com
Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Iron is very easily oxidized under alkaline conditions. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of.
AS Chemistry Redox Reactions and Group 2 Elements Owlcation
Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as. Iron Oxidation Energy.
From www.tf.uni-kiel.de
Free Enthalpy of Reduction or Oxidation Processes Iron Oxidation Energy The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Biogeochemical cycling of iron is crucial. Iron Oxidation Energy.
From owlcation.com
AS Chemistry Redox Reactions and Group 2 Elements Owlcation Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The total amount of energy produced by an electrochemical. Iron Oxidation Energy.
From www.researchgate.net
Model of iron oxidation in L. ferrodiazotrophum Direct oxidation occurs Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology,. Iron Oxidation Energy.
From encyclopedia.pub
Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds.. Iron Oxidation Energy.
From www.mccrone.com
Application Note Analysis of Iron Oxidation States by XPS Iron Oxidation Energy The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. In principle, iron oxidation can fuel significant primary productivity and nutrient. Iron Oxidation Energy.
From drawittoknowit.com
Biochemistry Glossary Oxidative Phosphorylation Draw It to Know It Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The total amount of energy. Iron Oxidation Energy.
From www.researchgate.net
Model of iron oxidation in F. acidarmanus Oxidation of iron is via a Iron Oxidation Energy Iron is very easily oxidized under alkaline conditions. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to. Iron Oxidation Energy.
From www.compoundchem.com
Compound Interest The Periodic Table of Oxidation States Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do. Iron Oxidation Energy.
From www.landscapedna.org
LandscapeDNA Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The oxidation state of an element is related to the number of electrons that an atom loses, gains,. Iron Oxidation Energy.
From sciencenotes.org
What Is Oxidation? Definition and Examples Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The total amount of energy produced by an. Iron Oxidation Energy.
From chemistry-europe.onlinelibrary.wiley.com
Combining Ligand Deuteration with Ligand Bulkiness in Non‐Heme Iron Iron Oxidation Energy The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Iron is very easily oxidized under alkaline conditions. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Oxygen. Iron Oxidation Energy.
From www.mccrone.com
Application Note Analysis of Iron Oxidation States by XPS Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. In principle, iron. Iron Oxidation Energy.
From www.showme.com
ShowMe oxidation state of CuCl2 Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became. Iron Oxidation Energy.
From pubs.rsc.org
Significance of anaerobic oxidation of methane (AOM) in mitigating Iron Oxidation Energy The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. Oxygen in the air oxidizes the iron(ii) hydroxide. Iron Oxidation Energy.
From marketbusinessnews.com
Oxidation definition and meaning Market Business News Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. Qiang li, yibo he, biao tang,. Iron Oxidation Energy.
From www.researchgate.net
Diagrams of occurrence of biotic and abiotic processes in iron Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Biogeochemical cycling of iron is crucial to. Iron Oxidation Energy.
From www.mccrone.com
Application Note Analysis of Iron Oxidation States by XPS Iron Oxidation Energy Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the. Iron Oxidation Energy.
From www.tessshebaylo.com
Half Equations For Rusting Of Iron Tessshebaylo Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical. Iron Oxidation Energy.
From www.dreamstime.com
Rust Formation and Iron Oxide Chemical Cause Explanation Outline Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Iron is very easily oxidized under alkaline conditions. Oxygen in the air oxidizes the iron(ii). Iron Oxidation Energy.
From www.biologynotes.site
OXIDATIVE PHOSPHORYLATION, ELECTRON TRANSPORT CHAIN — Biology Notes Iron Oxidation Energy Iron is very easily oxidized under alkaline conditions. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The different crystalline forms of iron oxide have found fertile ground. Iron Oxidation Energy.
From www.researchgate.net
Dependences of released, absorbed energy and heat of iron oxidation Iron Oxidation Energy Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining. Iron Oxidation Energy.
From quizlet.com
This oxidationreduction reaction in acidic solution is spon Quizlet Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. The oxidation state of an. Iron Oxidation Energy.
From www.researchgate.net
Iron oxidation states and atomic ratio obtained from Mössbauer Iron Oxidation Energy The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to. Iron Oxidation Energy.
From www.researchgate.net
3 Diagram showing the oxidation of Iron above 570 • C Download Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or. Iron Oxidation Energy.
From water-oxidation.blogspot.com
Artificial Photosynthesis chemical solutions for solar energy Iron Oxidation Energy The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under. Iron Oxidation Energy.
From www.mdpi.com
Hydrology Free FullText Iron and Manganese Oxidation States Iron Oxidation Energy The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore,. Iron Oxidation Energy.
From www.ahajournals.org
Mitochondrial Function, Skeletal Muscle Metabolism, and Iron Deficiency Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. The total amount of energy produced by an electrochemical cell, and thus the amount of energy. Iron Oxidation Energy.
From www.researchgate.net
Ironoxygen phase diagram [17]. Download Scientific Diagram Iron Oxidation Energy The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Qiang li, yibo he, biao tang, mingming li, zuoliang zhang & zongshu zou. Iron is very easily oxidized under alkaline conditions. The total amount of energy produced by. Iron Oxidation Energy.
From www.levelshealth.com
What is oxidative stress and why does it matter to metabolic health Iron Oxidation Energy Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. Iron is very easily oxidized under alkaline conditions. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The. Iron Oxidation Energy.
From ar.inspiredpencil.com
Example Of Oxidation Iron Oxidation Energy The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The different crystalline forms of iron oxide have found. Iron Oxidation Energy.
From www.semanticscholar.org
Figure 2 from Oxidation state and chemical shift investigation in Iron Oxidation Energy In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to. Iron Oxidation Energy.
From www.pnas.org
Ammonia oxidation pathways and nitrifier denitrification are Iron Oxidation Energy The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The different crystalline forms of iron oxide have found fertile ground in the field of nanotechnology, and therefore, became popular. The total amount of energy produced by an electrochemical cell, and. Iron Oxidation Energy.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Iron Oxidation Energy The oxidation state of an element is related to the number of electrons that an atom loses, gains, or appears to use when joining with another atom in compounds. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends. Iron is very easily oxidized under alkaline conditions. In. Iron Oxidation Energy.
From www.researchgate.net
curves of iron oxidation by atomic oxygen (4.82 × 10 16 at cm Iron Oxidation Energy Biogeochemical cycling of iron is crucial to many environmental processes, such as ocean productivity, carbon storage, greenhouse gas emissions and the fate of. Oxygen in the air oxidizes the iron(ii) hydroxide precipitate to iron(iii) hydroxide. In principle, iron oxidation can fuel significant primary productivity and nutrient cycling in dark environments such as the deep. Iron is very easily oxidized under. Iron Oxidation Energy.