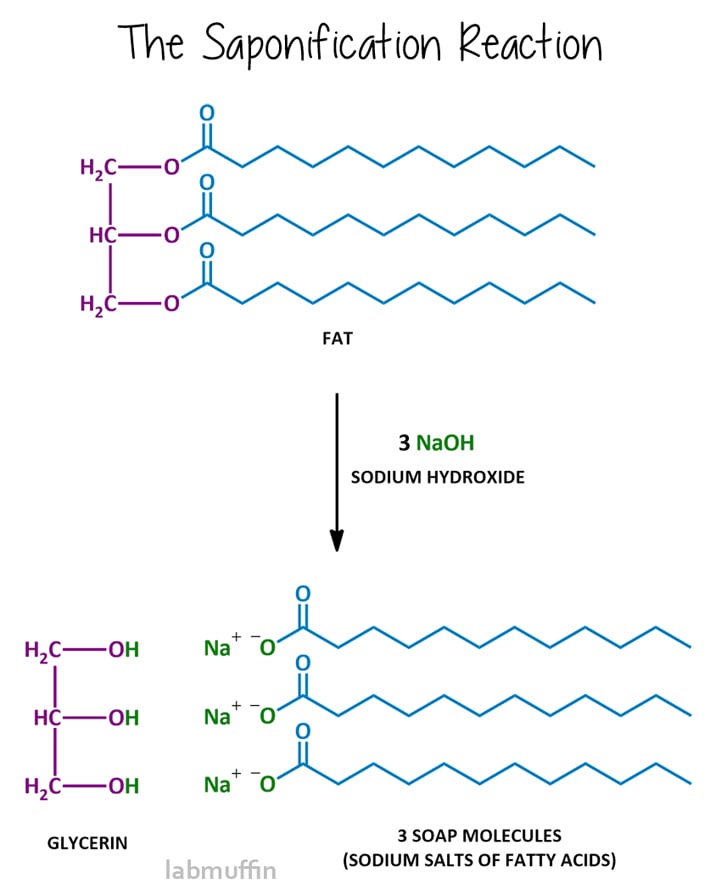
Making Soap Chemistry Lab . Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The ancient roman tradition called for mixing rain water,. You will precipitate the soap by. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. Not only is it a process that uses science,. The objective of this laboratory is to make lye soap via the saponification reaction.
from labmuffin.com
Not only is it a process that uses science,. You will precipitate the soap by. The objective of this laboratory is to make lye soap via the saponification reaction. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. For making detergent by sulfonation, the. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The ancient roman tradition called for mixing rain water,. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high.
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab
Making Soap Chemistry Lab Not only is it a process that uses science,. The ancient roman tradition called for mixing rain water,. You will precipitate the soap by. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The objective of this laboratory is to make lye soap via the saponification reaction. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide.
From www.youtube.com
Saponification Making Soap YouTube Making Soap Chemistry Lab You will precipitate the soap by. For making detergent by sulfonation, the. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The objective of this laboratory. Making Soap Chemistry Lab.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The ancient roman tradition called for mixing rain water,. Slowly, a chemical. Making Soap Chemistry Lab.
From learnphysics-dhruv.blogspot.com
Physics Learn To prepare Soap by cold process. Science practical GSEB Making Soap Chemistry Lab In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The objective of this laboratory is to make lye soap via the saponification reaction. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. For making detergent by sulfonation, the.. Making Soap Chemistry Lab.
From www.slideshare.net
Chemistry of soaps Making Soap Chemistry Lab The objective of this laboratory is to make lye soap via the saponification reaction. For making detergent by sulfonation, the. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. The science behind soap making is in the structure of the fats, the properties of the lye, and the. Making Soap Chemistry Lab.
From gioztfmla.blob.core.windows.net
Lab Report For Preparation Of Soap at Jessie Pardo blog Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. You will precipitate the soap by. Not only is it a process that uses science,. Slowly, a. Making Soap Chemistry Lab.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The ancient roman tradition called for mixing rain water,. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. In this experiment, you will make soap from. Making Soap Chemistry Lab.
From www.slideshare.net
Foaming Capacity of Soap Making Soap Chemistry Lab The objective of this laboratory is to make lye soap via the saponification reaction. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces. Making Soap Chemistry Lab.
From www.youtube.com
The Epic Chemistry of Soap YouTube Making Soap Chemistry Lab Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The science behind soap making is in the structure of the fats, the properties of the. Making Soap Chemistry Lab.
From crusadernews.com
Chemistry students design, create soaps Crusader News Making Soap Chemistry Lab Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. In this experiment, you will make soap from a. Making Soap Chemistry Lab.
From www.myxxgirl.com
Lab Synthesis Of Soap Pdf Laboratory Saponification And Soaps My XXX Making Soap Chemistry Lab For making detergent by sulfonation, the. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. The objective of this laboratory is to make lye soap via the saponification reaction. Not only is it a process that uses science,. In this experiment, you will make soap from a fat. Making Soap Chemistry Lab.
From www.thoughtco.com
How Saponification Makes Soap Making Soap Chemistry Lab For making detergent by sulfonation, the. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Not only is it a process that uses science,. The objective of this laboratory is. Making Soap Chemistry Lab.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Making Soap Chemistry Lab The ancient roman tradition called for mixing rain water,. Not only is it a process that uses science,. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse. Making Soap Chemistry Lab.
From giotostre.blob.core.windows.net
Saponification Soap Making Lye at Doreen Friend blog Making Soap Chemistry Lab Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. The ancient roman tradition called for mixing rain water,. You will precipitate the soap by. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Not only is it. Making Soap Chemistry Lab.
From www.instructables.com
Making Soap With Chemistry!! 7 Steps (with Pictures) Instructables Making Soap Chemistry Lab The ancient roman tradition called for mixing rain water,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. For making detergent by sulfonation, the. The science behind. Making Soap Chemistry Lab.
From giotostre.blob.core.windows.net
Saponification Soap Making Lye at Doreen Friend blog Making Soap Chemistry Lab For making detergent by sulfonation, the. The objective of this laboratory is to make lye soap via the saponification reaction. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide.. Making Soap Chemistry Lab.
From gioztfmla.blob.core.windows.net
Lab Report For Preparation Of Soap at Jessie Pardo blog Making Soap Chemistry Lab Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. The objective of this laboratory is to make lye soap via the saponification reaction. For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the. Making Soap Chemistry Lab.
From ch.pinterest.com
Pin på Toys Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. You will precipitate the soap by. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Slowly, a chemical. Making Soap Chemistry Lab.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog Making Soap Chemistry Lab The objective of this laboratory is to make lye soap via the saponification reaction. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap is. Making Soap Chemistry Lab.
From mugeek.vidalondon.net
Chemical Makeup Of Soap Mugeek Vidalondon Making Soap Chemistry Lab The ancient roman tradition called for mixing rain water,. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. After completing parts 1 and 2. Making Soap Chemistry Lab.
From www.pinterest.com
Pin em Ciencias Naturales Equipamento de laboratório, Laboratório de Making Soap Chemistry Lab For making detergent by sulfonation, the. Not only is it a process that uses science,. You will precipitate the soap by. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The ancient roman tradition called for mixing rain water,. Slowly, a chemical reaction called saponification would take place between. Making Soap Chemistry Lab.
From scienceexperimentsanimated.blogspot.com
Science fair Experiments Soaps and detergents for std 8 to 10 Science Making Soap Chemistry Lab The objective of this laboratory is to make lye soap via the saponification reaction. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. You will precipitate the soap by. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction. Making Soap Chemistry Lab.
From www.slideserve.com
PPT The Science of Soap PowerPoint Presentation, free download ID Making Soap Chemistry Lab In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Not only is it a process that uses science,. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Soap is made from reacting a fat. Making Soap Chemistry Lab.
From www.youtube.com
Preparation of soap Chemistry 3d Animation Studious YouTube Making Soap Chemistry Lab The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. After completing parts 1 and 2 of this experiment, rinse all glassware in the. Making Soap Chemistry Lab.
From steemkr.com
Saponification 1/2 — Steemkr Making Soap Chemistry Lab You will precipitate the soap by. Not only is it a process that uses science,. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The ancient roman tradition called for mixing rain water,. The science behind soap making is in the structure of the fats, the properties of the. Making Soap Chemistry Lab.
From learningfullriding.z14.web.core.windows.net
Science Of Soap Making Making Soap Chemistry Lab Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. For making detergent by sulfonation, the. The objective of this laboratory is to make lye soap via the saponification reaction. Not only is it a process that uses science,. Soap is made from reacting a fat or oil (or. Making Soap Chemistry Lab.
From gioztfmla.blob.core.windows.net
Lab Report For Preparation Of Soap at Jessie Pardo blog Making Soap Chemistry Lab Not only is it a process that uses science,. The ancient roman tradition called for mixing rain water,. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The objective of this laboratory is to make lye soap via the saponification reaction. After completing parts 1 and 2 of this. Making Soap Chemistry Lab.
From www.youtube.com
Lab 10 Part 3 Synthesis of Soap YouTube Making Soap Chemistry Lab The objective of this laboratory is to make lye soap via the saponification reaction. You will precipitate the soap by. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The ancient roman tradition called for mixing rain water,. For making detergent by sulfonation, the.. Making Soap Chemistry Lab.
From www.chemedx.org
Soap Making Chemical Education Xchange Making Soap Chemistry Lab Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The objective of this laboratory is to make lye soap via the saponification reaction. Not. Making Soap Chemistry Lab.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Making Soap Chemistry Lab For making detergent by sulfonation, the. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. The ancient roman tradition called for mixing rain water,. The science. Making Soap Chemistry Lab.
From worksheetdbbring.z21.web.core.windows.net
Science Of Soap Making Making Soap Chemistry Lab The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. The ancient roman tradition called for mixing rain water,. Not only is it a process that uses science,. After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with. Making Soap Chemistry Lab.
From www.facebook.com
Soap Chemistry Lab Making Soap Chemistry Lab For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. You will precipitate the soap by. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. After completing parts 1. Making Soap Chemistry Lab.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The objective of this laboratory is to make lye soap via the saponification reaction. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces. Making Soap Chemistry Lab.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Making Soap Chemistry Lab After completing parts 1 and 2 of this experiment, rinse all glassware in the laboratory sink with soap solution and rinse completely with. The ancient roman tradition called for mixing rain water,. You will precipitate the soap by. For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the. Making Soap Chemistry Lab.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Making Soap Chemistry Lab For making detergent by sulfonation, the. The science behind soap making is in the structure of the fats, the properties of the lye, and the chemical reaction that produces cleaning molecules. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. Soap is made from reacting a fat or. Making Soap Chemistry Lab.
From brainly.in
what is the process to make soap ? Brainly.in Making Soap Chemistry Lab Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. You will precipitate the soap by. Not only is it a process that uses science,. In this experiment, you will make soap from a fat or an oil by heating it with sodium hydroxide. Soap is made from reacting. Making Soap Chemistry Lab.