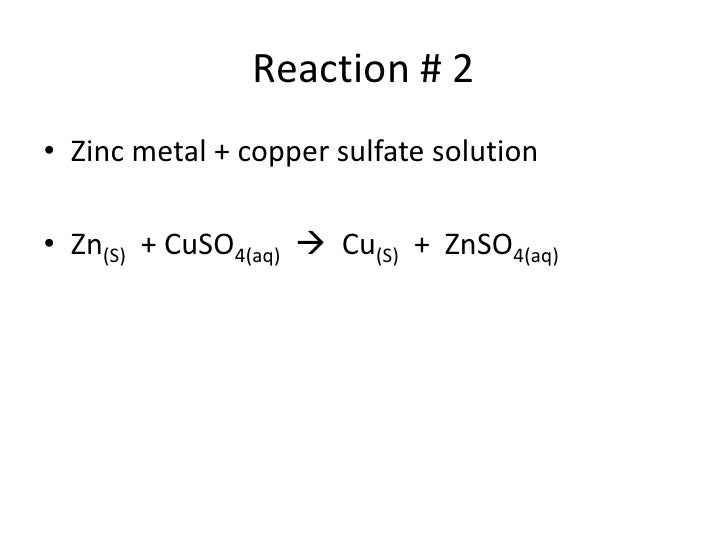
Copper And Zinc Sulfate Balanced Equation . Balancing step by step using the inspection method. Zn + cu {+2} = zn {+2} + cu. Let's balance this equation using the. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. What happens when a piece of zinc metal is added to copper sulphate solution? Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. In this lab you will. Also, write the balanced chemical equation if the reaction.
from www.slideshare.net
Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. In this lab you will. What happens when a piece of zinc metal is added to copper sulphate solution? Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cu {+2} = zn {+2} + cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Let's balance this equation using the. Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Also, write the balanced chemical equation if the reaction.
Replacement reactions
Copper And Zinc Sulfate Balanced Equation Let's balance this equation using the. Let's balance this equation using the. In this lab you will. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Balancing step by step using the inspection method. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zn + cu {+2} = zn {+2} + cu. Also, write the balanced chemical equation if the reaction. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. What happens when a piece of zinc metal is added to copper sulphate solution? Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate.
From tutorsuhu.com
3 What Is The Chemical Formula Of Copper Ii Sulfate Tutor Suhu Copper And Zinc Sulfate Balanced Equation Zn + cu {+2} = zn {+2} + cu. Let's balance this equation using the. What happens when a piece of zinc metal is added to copper sulphate solution? Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc. Copper And Zinc Sulfate Balanced Equation.
From www.transtutors.com
(Solved) Determine The Balanced Equation For The Precipitation Reaction... (1 Answer Copper And Zinc Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when a piece. Copper And Zinc Sulfate Balanced Equation.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe the... Course Hero Copper And Zinc Sulfate Balanced Equation Also, write the balanced chemical equation if the reaction. Balancing step by step using the inspection method. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cuso4 = znso4 + cu is a single. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Also, write the balanced chemical equation if the. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to balance zinc copper sulphate YouTube Copper And Zinc Sulfate Balanced Equation Also, write the balanced chemical equation if the reaction. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Balancing step by. Copper And Zinc Sulfate Balanced Equation.
From www.slideshare.net
Replacement reactions Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Let's balance this equation using the. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). An animation that shows the process at the particle level for the reaction between zinc and copper. Copper And Zinc Sulfate Balanced Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Copper And Zinc Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. What happens when a piece of zinc metal is added to copper sulphate solution? Also, write. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equation, including the states, for the reaction between Copper And Zinc Sulfate Balanced Equation In this lab you will. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zn + cu {+2} = zn {+2} + cu. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Balancing step by step using the inspection method. Multiplying. Copper And Zinc Sulfate Balanced Equation.
From www.toppr.com
The balanced equation for the following reactionCopper sulphate solution is added to sodium Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. In this lab you will. Also, write the balanced chemical equation if the reaction. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cu {+2} = zn {+2} + cu. An animation that shows. Copper And Zinc Sulfate Balanced Equation.
From www.toppr.com
Write balanced chemical equations for the following word equationCopper + Oxygen → Copper (II Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Multiplying the coefficient and. Copper And Zinc Sulfate Balanced Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Reaction of Zinc with Copper And Zinc Sulfate Balanced Equation Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. In this lab you will. Also, write the balanced chemical equation if the reaction. Let's balance this equation using the. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Multiplying the coefficient and the subscript of an element must. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Write the Formula for Copper (I) sulfate YouTube Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Let's balance this equation using the. Zn +. Copper And Zinc Sulfate Balanced Equation.
From www.slideshare.net
Replacement reactions Copper And Zinc Sulfate Balanced Equation Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. What happens when a piece of zinc metal is added to copper sulphate solution? In this lab you will. Also, write the balanced chemical equation if the reaction. An animation that shows the process at the particle level for the reaction between zinc. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
H2SO4+Zn=ZnSO4+H2 Balanced EquationSulphuric acid+Zinc=Zinc sulphate+Hydrogen Balanced Copper And Zinc Sulfate Balanced Equation What happens when a piece of zinc metal is added to copper sulphate solution? In this lab you will. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced molecular chemical equation for the reaction in aqueous solution for Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. In this lab you will. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Let's balance this equation using the. Balancing step by step using the inspection method. An animation that shows the process at the particle. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for the singlereplacement oxidationreduction reaction Copper And Zinc Sulfate Balanced Equation Balancing step by step using the inspection method. Let's balance this equation using the. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cu {+2} = zn {+2} + cu. Multiplying the coefficient and the subscript. Copper And Zinc Sulfate Balanced Equation.
From dxoqniotj.blob.core.windows.net
Copper Sulfate Formula And Charge at Rachel Bray blog Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zn + cu {+2} = zn {+2} + cu. In this lab you will. Let's balance this equation using the. Multiplying the coefficient and the subscript of an element must yield the same result on. Copper And Zinc Sulfate Balanced Equation.
From www.chegg.com
Solved Reactants Observations Zinc +copper(II) sulfate Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zn + cu {+2} = zn {+2} + cu. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. An animation that shows the. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. What happens when a piece of zinc metal is added to copper sulphate solution? In this. Copper And Zinc Sulfate Balanced Equation.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode during the Electrolysis of Copper And Zinc Sulfate Balanced Equation Also, write the balanced chemical equation if the reaction. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = znso4 + cu is a single displacement. Copper And Zinc Sulfate Balanced Equation.
From www.slideshare.net
Redox= quiz part 1 with answers Copper And Zinc Sulfate Balanced Equation Zn + cu {+2} = zn {+2} + cu. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Also, write the balanced chemical equation if the reaction. Balancing step by step using the inspection method. Multiplying the coefficient and the subscript of an element must yield the same result on. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations 1 zinc react with sulphate and hydrogen Copper And Zinc Sulfate Balanced Equation In this lab you will. Let's balance this equation using the. Also, write the balanced chemical equation if the reaction. Balancing step by step using the inspection method. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cuso4 = znso4 + cu is a. Copper And Zinc Sulfate Balanced Equation.
From cristianmeowterry.blogspot.com
Molecular Equation for Copper Ii Sulfate and Sodium Phosphate Copper And Zinc Sulfate Balanced Equation What happens when a piece of zinc metal is added to copper sulphate solution? Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Also, write the balanced chemical equation if the reaction. Zn + cu {+2} = zn {+2} + cu. Zinc. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Fe + CuSO4 = Fe2(SO4)3 + Cu YouTube Copper And Zinc Sulfate Balanced Equation What happens when a piece of zinc metal is added to copper sulphate solution? Balancing step by step using the inspection method. In this lab you will. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Let's balance this equation using the. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Multiplying the coefficient and. Copper And Zinc Sulfate Balanced Equation.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Photo Library Copper And Zinc Sulfate Balanced Equation Balancing step by step using the inspection method. What happens when a piece of zinc metal is added to copper sulphate solution? Also, write the balanced chemical equation if the reaction. Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of. Copper And Zinc Sulfate Balanced Equation.
From www.toppr.com
Write a balanced equation for the preparation of the following salts.(i) Copper sulphate from Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Let's balance this equation using the. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zn + cu {+2} = zn {+2} +. Copper And Zinc Sulfate Balanced Equation.
From www.toppr.com
5. What happens when a] Zinc reacts with Copper Sulphate solution. b) Aluminium reacts with Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. What happens when a piece of zinc metal is added to copper sulphate solution? In this lab you will. Also, write the balanced chemical. Copper And Zinc Sulfate Balanced Equation.
From www.tessshebaylo.com
Write A Balanced Chemical Equation For The Of Copper Ii Sulfate Pentahydrate Copper And Zinc Sulfate Balanced Equation Also, write the balanced chemical equation if the reaction. Let's balance this equation using the. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zn + cuso4 = znso4. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED B) zinc and copper(II) sulfate Obs Reagents And Srav Products And mlm reaction Reaction Copper And Zinc Sulfate Balanced Equation Balancing step by step using the inspection method. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Let's balance this equation using the. Zn + cuso4 = znso4 +. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper And Zinc Sulfate Balanced Equation An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Let's balance this equation using the. Zn + cu {+2} = zn {+2} + cu. Balancing step by step using the inspection method. What happens when a piece of zinc metal is added to copper sulphate solution? Zn + cuso4 =. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate) YouTube Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Let's balance this equation using the. Balancing step by step using the inspection method. An animation that shows the process at the particle level for the reaction between zinc and copper (ii) sulfate. Zn + cuso4 = cu2 +. Copper And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED REAcTIoN 124 COPPER SULFATE (Cuso,) AND AMMONIUM HYDROXIDE C (Write balanced equation Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. What happens when a piece of zinc metal is added to copper sulphate solution? Also, write the balanced chemical equation if the reaction. An animation that shows the process at the particle level for the reaction between zinc and. Copper And Zinc Sulfate Balanced Equation.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Copper And Zinc Sulfate Balanced Equation Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Balancing step by step using the inspection method. In this lab you will. Zn + cuso4 = znso4 + cu is a single displacement (substitution) reaction where one. Copper And Zinc Sulfate Balanced Equation.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part... Course Hero Copper And Zinc Sulfate Balanced Equation In this lab you will. Multiplying the coefficient and the subscript of an element must yield the same result on both sides of the balanced equation. Balancing step by step using the inspection method. What happens when a piece of zinc metal is added to copper sulphate solution? An animation that shows the process at the particle level for the. Copper And Zinc Sulfate Balanced Equation.
From ceotvsix.blob.core.windows.net
Magnesium And Zinc Sulfate Word Equation at Scott Winbush blog Copper And Zinc Sulfate Balanced Equation Zn + cuso4 = cu2 + znso4 is a single displacement (substitution). Zinc + cupric sulfate = dinuclear copper ion + zinc sulfate. Also, write the balanced chemical equation if the reaction. Let's balance this equation using the. Zn + cu {+2} = zn {+2} + cu. An animation that shows the process at the particle level for the reaction. Copper And Zinc Sulfate Balanced Equation.