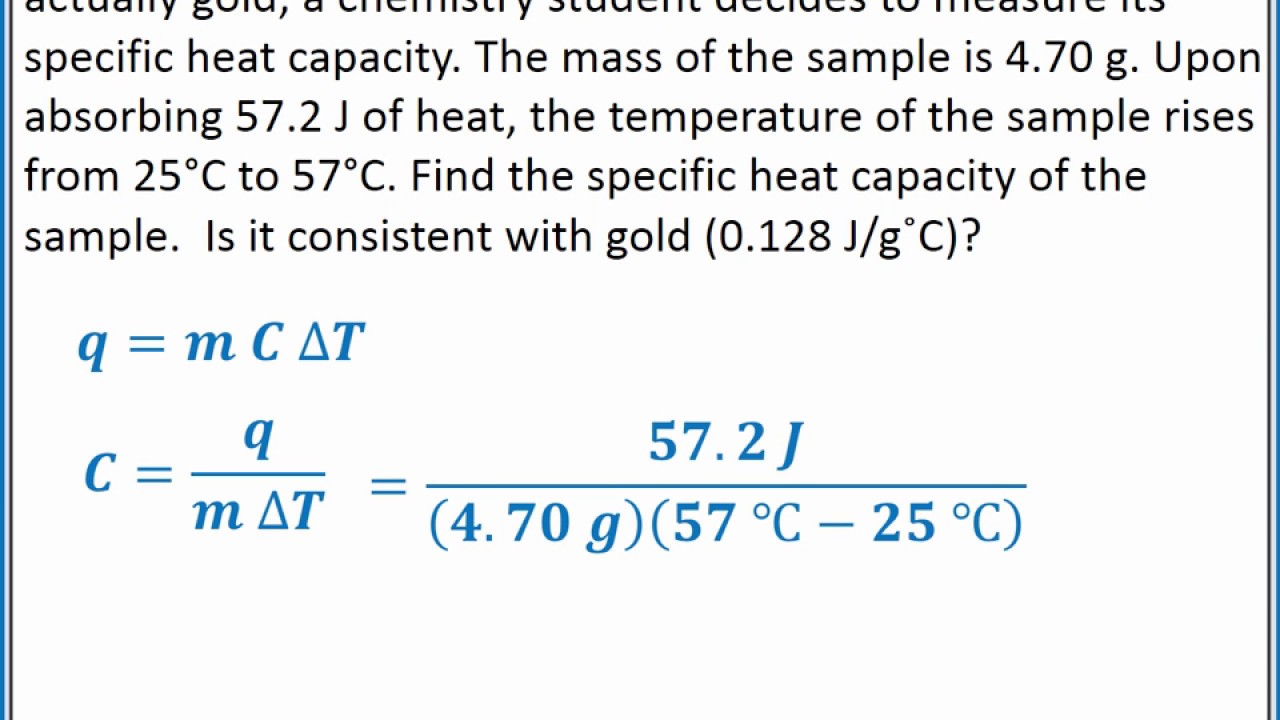
Specific Heat Capacity In Calorimetry . The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Apply the first law of thermodynamics to calorimetry. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The units of specific heat capacity are j/(kg °c) or. The specific heat is numerically equal to the. The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The symbol c stands for the.
from www.youtube.com
The specific heat is numerically equal to the. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The symbol c stands for the. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter.
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube
Specific Heat Capacity In Calorimetry The symbol c stands for the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The units of specific heat capacity are j/(kg °c) or. Apply the first law of thermodynamics to calorimetry. The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The specific heat is numerically equal to the. The symbol c stands for the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity, and Molar Heat Capacity Specific Heat Capacity In Calorimetry It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Compare heat flow from hot to cold. Specific Heat Capacity In Calorimetry.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Specific Heat Capacity In Calorimetry The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Compare heat flow from hot to cold objects in an ideal. Specific Heat Capacity In Calorimetry.
From www.youtube.com
Specific heat capacity YouTube Specific Heat Capacity In Calorimetry It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The symbol c stands for the. The specific heat is numerically equal to the. The units of specific heat capacity are j/(kg °c) or. Compare heat flow from hot to cold objects in an ideal. Specific Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 Specific Heat Capacity In Calorimetry The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. Apply the first law of thermodynamics to calorimetry. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The specific heat capacity is. Specific Heat Capacity In Calorimetry.
From studymind.co.uk
Heat Capacity Study Mind Specific Heat Capacity In Calorimetry Apply the first law of thermodynamics to calorimetry. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. It describes. Specific Heat Capacity In Calorimetry.
From electriccarheatingsystembijitsuke.blogspot.com
Electric Car Heating System Specific Heat Capacity Formula Specific Heat Capacity In Calorimetry The symbol c stands for the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Apply the first law of thermodynamics to calorimetry. The specific heat is numerically equal to the.. Specific Heat Capacity In Calorimetry.
From byjus.com
To Determine Specific Heat Capacity Of A Given Solid Physics Practical Specific Heat Capacity In Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The units of specific heat capacity are j/(kg °c) or. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt. Specific Heat Capacity In Calorimetry.
From askfilo.com
Calorimetry Meaning, Specific heat capacities, Principle of heat capaci.. Specific Heat Capacity In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The units of specific heat capacity are j/(kg °c) or. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The symbol c stands for the specific heat (also called “ specific. Specific Heat Capacity In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Specific Heat Capacity In Calorimetry Apply the first law of thermodynamics to calorimetry. The specific heat is numerically equal to the. The symbol c stands for the. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity. Specific Heat Capacity In Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry I Specific Heat Capacity In Calorimetry The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the.. Specific Heat Capacity In Calorimetry.
From www.youtube.com
Heat Capacity, Specific Heat, and Calorimetry YouTube Specific Heat Capacity In Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. Apply the first law of thermodynamics to calorimetry. The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c.. Specific Heat Capacity In Calorimetry.
From www.scribd.com
Determining Specific Heat Capacities through Calorimetry Experiments PDF Heat Heat Capacity Specific Heat Capacity In Calorimetry The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The specific heat capacity is the heat or energy required to change one. Specific Heat Capacity In Calorimetry.
From www.youtube.com
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples YouTube Specific Heat Capacity In Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The specific heat is numerically equal to the.. Specific Heat Capacity In Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Specific Heat Capacity In Calorimetry The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat is numerically equal to the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the. The units of specific heat capacity are j/(kg. Specific Heat Capacity In Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Specific Heat Capacity In Calorimetry The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The specific heat is numerically equal to the. The specific heat capacity is the. Specific Heat Capacity In Calorimetry.
From learningfullherman.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Capacity In Calorimetry The specific heat is numerically equal to the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Apply the first law of thermodynamics to calorimetry. It describes how much heat must be added to a unit of mass of a given. Specific Heat Capacity In Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Specific Heat Capacity In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends. Specific Heat Capacity In Calorimetry.
From studylib.net
Heat Equation Specific Heat Capacity In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The specific heat is numerically equal to the. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The symbol c stands for the. Q = mcδt, where q. Specific Heat Capacity In Calorimetry.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 Specific Heat Capacity In Calorimetry The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c. The units of specific heat capacity are j/(kg °c) or. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. Q = mcδt,. Specific Heat Capacity In Calorimetry.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Specific Heat Capacity In Calorimetry The specific heat is numerically equal to the. The units of specific heat capacity are j/(kg °c) or. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat capacity. Specific Heat Capacity In Calorimetry.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Specific Heat Capacity In Calorimetry Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat is numerically equal to the. The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1. Specific Heat Capacity In Calorimetry.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Specific Heat Capacity In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or. Specific Heat Capacity In Calorimetry.
From slidetodoc.com
CHEM 1011 Calorimetry The Determination of the Specific Specific Heat Capacity In Calorimetry The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. Apply the first law of thermodynamics to calorimetry. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The units of specific heat capacity are j/(kg. Specific Heat Capacity In Calorimetry.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie calorimeter joule specific heat Specific Heat Capacity In Calorimetry The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c. The units of specific heat capacity are j/(kg °c) or. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The specific heat. Specific Heat Capacity In Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Specific Heat Capacity In Calorimetry The units of specific heat capacity are j/(kg °c) or. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Apply the first law of thermodynamics to calorimetry. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the. Specific Heat Capacity In Calorimetry.
From studylib.net
Calorimetry Lab Specific Heat Capacity Specific Heat Capacity In Calorimetry The specific heat is numerically equal to the. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. The symbol c stands for the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Q = mcδt, where q is the symbol. Specific Heat Capacity In Calorimetry.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Specific Heat Capacity In Calorimetry It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Apply the first law of thermodynamics to calorimetry. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat is numerically. Specific Heat Capacity In Calorimetry.
From www.youtube.com
Heat capacity, Specific heat capacity, Calorimetry & Change of state, Live 4, Ch. 11, Heat, 11th Specific Heat Capacity In Calorimetry The specific heat capacity is the heat or energy required to change one unit mass of a substance of a constant volume by 1 °c. The units of specific heat capacity are j/(kg °c) or. The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. Apply the first. Specific Heat Capacity In Calorimetry.
From users.highland.edu
Calorimetry Specific Heat Capacity In Calorimetry The units of specific heat capacity are j/(kg °c) or. The symbol c stands for the. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of. Specific Heat Capacity In Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Specific Heat Capacity In Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The units of specific heat capacity are j/(kg °c) or. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the specific. Specific Heat Capacity In Calorimetry.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Specific Heat Capacity In Calorimetry The specific heat capacity (\(c\)) of a substance, commonly called its specific heat, is the quantity of heat required to raise the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The specific heat is numerically equal to the. Apply the. Specific Heat Capacity In Calorimetry.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Specific Heat Capacity In Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The symbol c stands for the. It describes how much heat must be added to a unit of mass of a given substance to raise its temperature by one degree celsius. The symbol c stands for the specific heat (also called “ specific heat. Specific Heat Capacity In Calorimetry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application Specific Heat Capacity In Calorimetry Apply the first law of thermodynamics to calorimetry. The units of specific heat capacity are j/(kg °c) or. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The symbol c stands for the. The specific heat is numerically equal to the. It describes how much heat must. Specific Heat Capacity In Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube Specific Heat Capacity In Calorimetry Apply the first law of thermodynamics to calorimetry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The units of. Specific Heat Capacity In Calorimetry.
From dokumen.tips
(PPTX) Calorimetry Calorimetry is used to measure heat capacity and specific heats. calorimeter Specific Heat Capacity In Calorimetry Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical reaction. The specific heat capacity (\(c\)) of a substance, commonly. Specific Heat Capacity In Calorimetry.