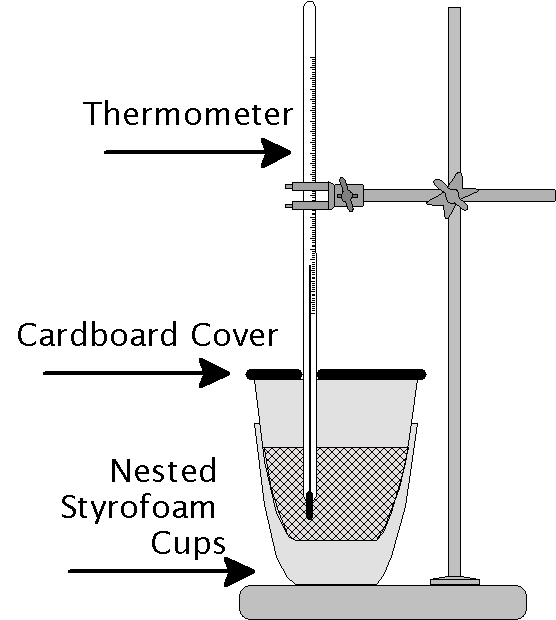
Calorimetry Lab Physics . Calorimeters can be used to find a substance’s specific heat. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry is a method used to measure heat from one material to another. Calorimetry professor karpf college physics 011 lab by: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • distinguish among these concepts: Before coming to lab you should be able to: Natalia kastsaridis 14 february 2021 introduction:
from kaffee.50webs.com
Before coming to lab you should be able to: Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimetry professor karpf college physics 011 lab by: Natalia kastsaridis 14 february 2021 introduction: Calorimetry is a method used to measure heat from one material to another. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. • distinguish among these concepts:
Lab Calorimetry
Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Before coming to lab you should be able to: Calorimetry is a method used to measure heat from one material to another. Natalia kastsaridis 14 february 2021 introduction: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry professor karpf college physics 011 lab by: Calorimeters can be used to find a substance’s specific heat. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • distinguish among these concepts:
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Lab Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimeters can be used to find a substance’s specific heat. Calorimetry is a method used to measure heat from one material to another. • distinguish among these concepts: Before coming. Calorimetry Lab Physics.
From studylib.net
Lab 12.1 Calorimetry KET Virtual Physics Labs Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. Calorimetry is a method used to measure heat from one material to another. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Experiment 6 ∙ calorimetry 6‐3 consider what. Calorimetry Lab Physics.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Physics When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • distinguish among these concepts: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Before coming to lab you should be. Calorimetry Lab Physics.
From www.youtube.com
Calorimeter 10th Std Physics ICSE Board Home Revise YouTube Calorimetry Lab Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry professor karpf college physics 011 lab by: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water. Calorimetry Lab Physics.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Calorimetry Lab Physics • distinguish among these concepts: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimeters can be used to find a substance’s specific heat. Before coming to lab you should be able to: When two bodies in an isolated. Calorimetry Lab Physics.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Lab Physics Calorimetry is a method used to measure heat from one material to another. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. When a hot. Calorimetry Lab Physics.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Lab Physics Calorimetry is a method used to measure heat from one material to another. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimeters can be. Calorimetry Lab Physics.
From www.studocu.com
Lab 9 Calorimetry (manual) PHYS 161 Lab 8 Calorimetry Abstract This Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Natalia kastsaridis 14 february 2021 introduction: Calorimetry is a method used to measure heat from one. Calorimetry Lab Physics.
From has.hc.edu.tw
3/3 Physics Calorimetry Lab Calorimetry Lab Physics Calorimetry is a method used to measure heat from one material to another. Calorimetry professor karpf college physics 011 lab by: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. Before coming to lab you. Calorimetry Lab Physics.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Lab Physics • distinguish among these concepts: Calorimetry is a method used to measure heat from one material to another. When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimeters can be used to find a substance’s specific heat. A container that prevents heat transfer in or out is called a. Calorimetry Lab Physics.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimetry Lab Physics Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimeters can be used to find a substance’s specific heat. Calorimetry is a method used to measure heat from one material to another. When a hot object is placed in the calorimeter, heat energy is transferred. Calorimetry Lab Physics.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Lab Physics Natalia kastsaridis 14 february 2021 introduction: • distinguish among these concepts: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Before coming to lab you should be able to: Calorimeters can be used to find a substance’s specific heat. Calorimetry professor karpf college physics 011. Calorimetry Lab Physics.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Natalia kastsaridis 14 february 2021 introduction: • distinguish among these concepts: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to. Calorimetry Lab Physics.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Lab Physics Calorimetry professor karpf college physics 011 lab by: Natalia kastsaridis 14 february 2021 introduction: Calorimetry is a method used to measure heat from one material to another. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Before coming to lab you should be able to:. Calorimetry Lab Physics.
From pressbooks.online.ucf.edu
1.4 Heat Transfer, Specific Heat, and Calorimetry General Physics Calorimetry Lab Physics Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Before coming to lab you should be able to: Calorimetry professor karpf college physics 011 lab by: Natalia kastsaridis 14 february 2021 introduction: Calorimetry is a method used to measure heat from one material to another.. Calorimetry Lab Physics.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. Natalia kastsaridis 14 february 2021 introduction: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and. Calorimetry Lab Physics.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Lab Physics Calorimetry is a method used to measure heat from one material to another. Calorimeters can be used to find a substance’s specific heat. Natalia kastsaridis 14 february 2021 introduction: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. •. Calorimetry Lab Physics.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry professor karpf college physics 011 lab by: When a. Calorimetry Lab Physics.
From studylib.net
Calorimetry and Coffee Cups Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Before coming to lab you should be able to: When a hot object is placed in. Calorimetry Lab Physics.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry professor karpf college physics 011 lab by: • distinguish. Calorimetry Lab Physics.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimetry professor karpf college physics 011 lab by: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Before coming to lab you should be able. Calorimetry Lab Physics.
From www.japson.com
Joule's Calorimeter Heat Physics Lab Products Products Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. Before coming to lab you should be able to: Natalia kastsaridis 14 february 2021 introduction: • distinguish among these concepts: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. When two bodies in an isolated. Calorimetry Lab Physics.
From users.highland.edu
Calorimetry Calorimetry Lab Physics Before coming to lab you should be able to: • distinguish among these concepts: Natalia kastsaridis 14 february 2021 introduction: Calorimetry professor karpf college physics 011 lab by: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimetry is a method used to measure heat from one material to. Calorimetry Lab Physics.
From ar.inspiredpencil.com
Calorimeter Calorimetry Lab Physics Calorimetry professor karpf college physics 011 lab by: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. • distinguish among these concepts: Calorimeters can be. Calorimetry Lab Physics.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Before coming to lab you should be able to: Calorimetry professor karpf college physics 011 lab by: Natalia kastsaridis 14 february 2021 introduction: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into. Calorimetry Lab Physics.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Calorimetry Lab Physics Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry is a method used to measure heat from one material to another. Natalia kastsaridis 14 february 2021 introduction: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with. Calorimetry Lab Physics.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. • distinguish among these concepts: A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is a method used to measure heat from one material to another. When a. Calorimetry Lab Physics.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Natalia kastsaridis. Calorimetry Lab Physics.
From www.studocu.com
Calorimetry Lab Report Physics 212 General Physics With Calculus 212 Calorimetry Lab Physics Natalia kastsaridis 14 february 2021 introduction: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimeters can be used to find a substance’s specific heat. • distinguish among these concepts: Before coming to lab you should be able to: Calorimetry is a method used to measure heat from one. Calorimetry Lab Physics.
From 2012books.lardbucket.org
Calorimetry Calorimetry Lab Physics Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Natalia kastsaridis 14 february 2021 introduction:. Calorimetry Lab Physics.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a. Calorimetry professor karpf college physics 011 lab by: Calorimeters can be used to find a substance’s. Calorimetry Lab Physics.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Lab Physics When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Natalia kastsaridis 14 february 2021 introduction: Calorimetry is a method used to measure heat from one material to another. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold. Calorimetry Lab Physics.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Lab Physics When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • distinguish among these concepts: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Calorimetry professor karpf college physics 011 lab by: Natalia kastsaridis 14. Calorimetry Lab Physics.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. • distinguish among these concepts: Experiment. Calorimetry Lab Physics.
From physicswithfong.wordpress.com
Day 8 CALORIMETRY LAB PhysicswithFong By Natalie Tegart Calorimetry Lab Physics Calorimeters can be used to find a substance’s specific heat. Calorimetry professor karpf college physics 011 lab by: • distinguish among these concepts: Natalia kastsaridis 14 february 2021 introduction: When two bodies in an isolated system, initially at different temperatures, are placed in intimate contact with each other, in. Before coming to lab you should be able to: Experiment 6. Calorimetry Lab Physics.