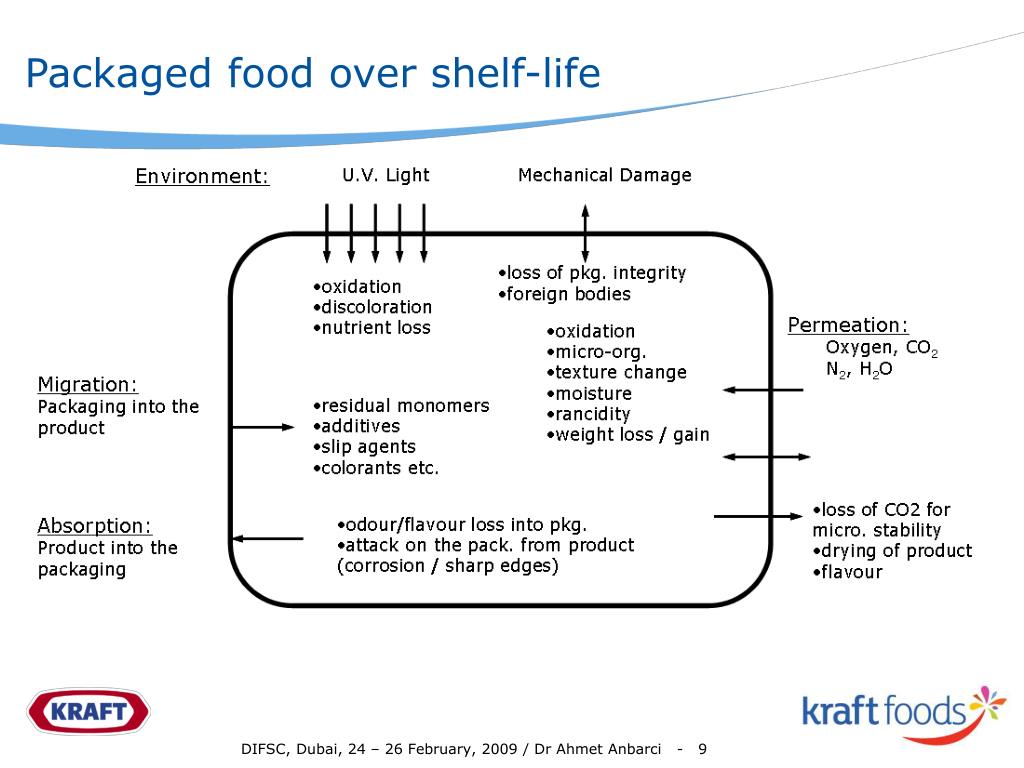
Shelf Life Definition Ich . the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. the international conference on harmonisation (ich) of technical. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. The retest period or shelf life proposed should not exceed that predicted for any single. the current international council for harmonisation of technical requirements for pharmaceuticals for. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest.
from www.slideserve.com
this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. The retest period or shelf life proposed should not exceed that predicted for any single. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the current international council for harmonisation of technical requirements for pharmaceuticals for. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical.
PPT ShelfLife of Prepackaged Food Products An Industry Perspective
Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. The retest period or shelf life proposed should not exceed that predicted for any single. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the international conference on harmonisation (ich) of technical. the current international council for harmonisation of technical requirements for pharmaceuticals for. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in.
From www.slideserve.com
PPT Food Regulations PowerPoint Presentation, free download ID4022666 Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. The retest period or shelf life proposed should not exceed that predicted for any single. this guidance provides recommendations on how to use. Shelf Life Definition Ich.
From www.slideserve.com
PPT ShelfLife of Prepackaged Food Products An Industry Perspective Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. the international conference on harmonisation (ich) of technical. the current international council for harmonisation of technical requirements for pharmaceuticals for. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. this document explains. Shelf Life Definition Ich.
From www.slideserve.com
PPT Shelf Life Practice Questions PowerPoint Presentation, free Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical. the current international council for harmonisation of technical requirements for pharmaceuticals for. the following guideline. Shelf Life Definition Ich.
From www.alamy.com
Extended shelf life blue gradient concept icon Stock Vector Image & Art Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2). Shelf Life Definition Ich.
From antiqueshelfbracketsid.blogspot.com
Shelf Life Definition Antique Shelf Brackets Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. the current international council for harmonisation of technical requirements. Shelf Life Definition Ich.
From www.youtube.com
Shelf life Meaning YouTube Shelf Life Definition Ich this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The retest period or shelf life proposed should not exceed that predicted for any single. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. cases, for example,. Shelf Life Definition Ich.
From foodresearchlab.com
Food Research Lab What are the different methods the Food Shelf Life Definition Ich this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the current international council for harmonisation of technical requirements for pharmaceuticals for. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. the following guideline is a. Shelf Life Definition Ich.
From www.reddit.com
A cool guide about Shelf life after best before date r/coolguides Shelf Life Definition Ich this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The retest period or shelf life proposed should not exceed that predicted for any single. the international conference on harmonisation (ich) of technical. this guidance provides recommendations on how to use stability data generated in accordance. Shelf Life Definition Ich.
From exoyxxesq.blob.core.windows.net
Shelf Life Definition In Medical Terminology at Kathryn Abernathy blog Shelf Life Definition Ich this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical. The retest period or shelf life proposed should not exceed that predicted for any single. cases, for example, for unstable apis, a shelf life is given) or a shelf life for. Shelf Life Definition Ich.
From brentwoodplastics.com
Factors Determining Shelf Life Brentwood Plastics Shelf Life Definition Ich the current international council for harmonisation of technical requirements for pharmaceuticals for. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. . Shelf Life Definition Ich.
From www.penguin.com.au
Shelf Life by Simon Parke Penguin Books Australia Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the international conference on harmonisation (ich) of technical. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the current international council. Shelf Life Definition Ich.
From www.slideserve.com
PPT Shelf Life PowerPoint Presentation, free download ID2067773 Shelf Life Definition Ich the current international council for harmonisation of technical requirements for pharmaceuticals for. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage. Shelf Life Definition Ich.
From www.davidwaynereed.com
Shelf Life — David Wayne Reed Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical. The retest period or shelf. Shelf Life Definition Ich.
From finwise.edu.vn
Albums 93+ Pictures What Is The Shelf Life Of 5.56 Ammo Updated Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the following guideline is a revised version of the ich q1a guideline and. Shelf Life Definition Ich.
From www.realmensow.co.uk
How To Properly Store Fertilizers Real Men Sow Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. The retest period or shelf. Shelf Life Definition Ich.
From exoyxxesq.blob.core.windows.net
Shelf Life Definition In Medical Terminology at Kathryn Abernathy blog Shelf Life Definition Ich this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the current international council for harmonisation of technical requirements for pharmaceuticals for. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. this document explains how. Shelf Life Definition Ich.
From q1scientific.com
Shelf life studies for Food Controlled Environmental Storage Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. The retest period or shelf life proposed should not exceed. Shelf Life Definition Ich.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID999616 Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. The retest period or shelf life proposed should not exceed that predicted for any single. the current international council for harmonisation of technical requirements for pharmaceuticals for. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. . Shelf Life Definition Ich.
From issuu.com
Shelf Life Cheat Sheet by Jon Dee Issuu Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the current international council for harmonisation of technical requirements for pharmaceuticals for. the following guideline is. Shelf Life Definition Ich.
From www.hyphen-stat.com
Shelflife computation Hyphenstat Shelf Life Definition Ich the current international council for harmonisation of technical requirements for pharmaceuticals for. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. The retest period or shelf life proposed should not exceed that predicted for any single. this guidance provides recommendations on how to use. Shelf Life Definition Ich.
From antiqueshelfbracketsid.blogspot.com
Shelf Life Definition Antique Shelf Brackets Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. The retest period or shelf. Shelf Life Definition Ich.
From rbitzer.com
The 411 on Food Safety and Sanitation Rebecca Bitzer & Associates Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical. the following guideline is. Shelf Life Definition Ich.
From www.gearaid.com
Adhesive Shelf Life GEAR AID Help Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. the current international council for harmonisation of technical requirements for pharmaceuticals for. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. the international conference on harmonisation (ich) of technical. cases, for example,. Shelf Life Definition Ich.
From www.alamy.com
Shelf Life High Resolution Stock Photography and Images Alamy Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. this guidance provides recommendations on how to use stability data. Shelf Life Definition Ich.
From mrlabel.com
Shelf Life of Stickers & Labels (Detailed Guide) Shelf Life Definition Ich the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. the current international council for harmonisation of technical requirements for pharmaceuticals for. the international conference on harmonisation (ich) of technical. this document explains how to use stability data generated in accordance with the ich. Shelf Life Definition Ich.
From shelfwithhooks.blogspot.com
Meaning Of Shelf Life Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the following guideline is. Shelf Life Definition Ich.
From www.youtube.com
Shelf life — what is SHELF LIFE meaning YouTube Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. the international conference on harmonisation (ich) of technical. this guidance provides recommendations on how to use stability data generated in. Shelf Life Definition Ich.
From loeaowjzm.blob.core.windows.net
Shelf Life Extension Eu at Blake Sutherland blog Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the. Shelf Life Definition Ich.
From www.linkedin.com
A Guide to Calculating the Shelf Life of Foods Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. The retest period or shelf life proposed should not exceed that predicted for any single. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. this guidance provides recommendations on how to use stability data generated in. Shelf Life Definition Ich.
From antiqueshelfbracketsid.blogspot.com
Shelf Life Definition Antique Shelf Brackets Shelf Life Definition Ich the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. The retest period or shelf life proposed should not exceed that predicted for any single. this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. cases, for. Shelf Life Definition Ich.
From www.slideserve.com
PPT ShelfLife of Prepackaged Food Products An Industry Perspective Shelf Life Definition Ich cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. The retest period or shelf life proposed should not exceed that. Shelf Life Definition Ich.
From www.alamy.com
Shelf Life High Resolution Stock Photography and Images Alamy Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. cases, for example, for unstable apis, a shelf life is given) or a shelf life for the fpp can be established and storage conditions can be. the international conference on harmonisation (ich) of technical. the current international council for harmonisation of technical. Shelf Life Definition Ich.
From www.scommerce-mage.com
Shelf Life extension for Magento 2 ERP Define Product Expiry Date Shelf Life Definition Ich The retest period or shelf life proposed should not exceed that predicted for any single. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. the international conference on harmonisation (ich) of technical. cases, for example, for unstable apis, a shelf life is given) or. Shelf Life Definition Ich.
From www.researchgate.net
(PDF) Penentuan umur simpan keripik buah dengan metode accelerated Shelf Life Definition Ich the international conference on harmonisation (ich) of technical. The retest period or shelf life proposed should not exceed that predicted for any single. the following guideline is a revised version of the ich q1a guideline and defines the stability data package for a new drug. this guidance provides recommendations on how to use stability data generated in. Shelf Life Definition Ich.
From latampharmara.com
Stability Studies Regulatory Affairs in Latin America Shelf Life Definition Ich this guidance provides recommendations on how to use stability data generated in accordance with the principles detailed in. this document explains how to use stability data generated in accordance with the ich guideline q1a (r2) to propose a retest. the current international council for harmonisation of technical requirements for pharmaceuticals for. cases, for example, for unstable. Shelf Life Definition Ich.