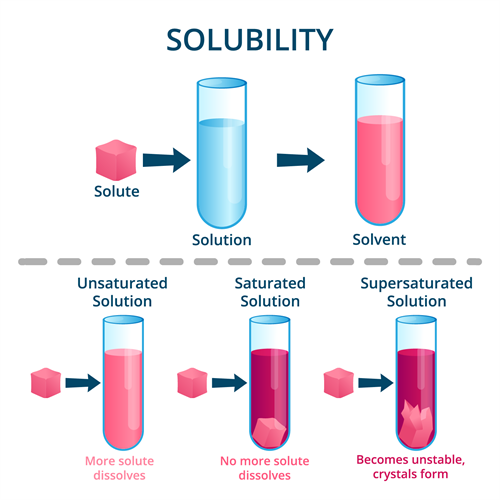
Example Of Unsaturated Solvent . Vinegar is an unsaturated solution of acetic. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Vinegar is an acetic acid solution in unsaturated water. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent.
from www.yaclass.in
Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Vinegar is an acetic acid solution in unsaturated water. Vinegar is an unsaturated solution of acetic. In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution.
Types of solutions Based on the amount of the solute — lesson. Science
Example Of Unsaturated Solvent An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Vinegar is an unsaturated solution of acetic. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Vinegar is an acetic acid solution in unsaturated water. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution.
From www.pinterest.com
Unsaturated Solution Easy Science Easy science, Solutions, Science Example Of Unsaturated Solvent Vinegar is an acetic acid solution in unsaturated water. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Apply a solubility conversion factor to. Example Of Unsaturated Solvent.
From www.youtube.com
Saturated Unsaturated and Supersaturated Solutions What is the Example Of Unsaturated Solvent In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of. Example Of Unsaturated Solvent.
From learningauttranadhie1h.z21.web.core.windows.net
Mixture And Solution Worksheets Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Vinegar is an acetic acid solution in unsaturated water. An unsaturated solution is a solution. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Separating solutions. PowerPoint Presentation, free download ID Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Vinegar is an acetic acid solution in unsaturated water. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. An unsaturated solution is a chemical solution in which the concentration of the solute. Example Of Unsaturated Solvent.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Example Of Unsaturated Solvent In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Vinegar is an acetic acid solution in unsaturated water. Apply a solubility conversion factor to calculate the amount. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Unsaturated Hydrocarbons PowerPoint Presentation, free download Example Of Unsaturated Solvent An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar is an unsaturated solution of acetic. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the. Example Of Unsaturated Solvent.
From www.youtube.com
Solutions Lesson 1 Solutions and Solubility YouTube Example Of Unsaturated Solvent Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act. Example Of Unsaturated Solvent.
From study.com
What is a Solution in Science? Definition & Examples Video & Lesson Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Vinegar is an acetic acid solution in unsaturated water. An unsaturated solution is a chemical solution in. Example Of Unsaturated Solvent.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated. Example Of Unsaturated Solvent.
From www.youtube.com
TYPES OF SOLUTIONS UNSATURATED, SATURATED, SUPERSATURATED GRADE 7 Example Of Unsaturated Solvent In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Vinegar is an acetic acid solution in unsaturated water. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). An unsaturated solution is a chemical. Example Of Unsaturated Solvent.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Example Of Unsaturated Solvent Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar is an unsaturated solution of acetic. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Any solution with a concentration of solute below the saturation point of the solute is. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Unit 7 Solution Chemistry Chapter 13 PowerPoint Presentation Example Of Unsaturated Solvent Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Vinegar is an acetic acid solution in unsaturated water. Unsaturated solutions are solutions in which the amount of. Example Of Unsaturated Solvent.
From www.slideshare.net
what is saturated,unsaturated and supersaturated solution Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Vinegar is an unsaturated solution of acetic. Any solution with a concentration of solute. Example Of Unsaturated Solvent.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Vinegar is an acetic acid solution in unsaturated water. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar is an unsaturated solution of acetic. In a specific. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Vinegar is. Example Of Unsaturated Solvent.
From www.teachoo.com
Difference between Saturated and Unsaturated Solution Teachoo Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar is an unsaturated solution of acetic. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Review Next 3 slides PowerPoint Presentation, free download ID Example Of Unsaturated Solvent Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Vinegar is an. Example Of Unsaturated Solvent.
From quizlet.com
Mixtures and Solutions Diagram Quizlet Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. An unsaturated. Example Of Unsaturated Solvent.
From sciencenotes.org
Supersaturated Solution Definition and Examples Example Of Unsaturated Solvent Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Vinegar is an acetic acid solution in unsaturated water. An unsaturated solution is a solution that contains less than. Example Of Unsaturated Solvent.
From www.youtube.com
Saturated And Unsaturated Solution, General Science Lecture Sabaq.pk Example Of Unsaturated Solvent In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Vinegar is an. Example Of Unsaturated Solvent.
From www.pinterest.co.uk
saturated unsaturated and supersaturated solutions Google Search Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific. Example Of Unsaturated Solvent.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Example Of Unsaturated Solvent Vinegar is an acetic acid solution in unsaturated water. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a solution that contains. Example Of Unsaturated Solvent.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Example Of Unsaturated Solvent An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Vinegar is an acetic acid solution in unsaturated water. Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Vinegar is an unsaturated solution of acetic. Adding a spoonful of sugar. Example Of Unsaturated Solvent.
From ar.inspiredpencil.com
Unsaturated Solution Example Of Unsaturated Solvent Vinegar is an unsaturated solution of acetic. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. In a specific gas mixture, the solute may be solid,. Example Of Unsaturated Solvent.
From www.geeksforgeeks.org
Saturated and Unsaturated Solutions Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. Apply a solubility conversion factor to calculate the amount of solute that can. Example Of Unsaturated Solvent.
From www.teachoo.com
[Class 10] What are saturated & unsaturated hydrocarbon with examples? Example Of Unsaturated Solvent An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Saturated, Unsaturated & Supersaturated PowerPoint Presentation Example Of Unsaturated Solvent Vinegar is an acetic acid solution in unsaturated water. Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). An unsaturated solution is a solution that contains. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Chapter 7 Solutions PowerPoint Presentation ID2121794 Example Of Unsaturated Solvent Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Vinegar is an acetic acid solution in unsaturated water. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Vinegar is an unsaturated solution of acetic. Any solution with. Example Of Unsaturated Solvent.
From www.ck12.org
Saturated, Unsaturated, Supersaturated Overview ( Video ) Chemistry Example Of Unsaturated Solvent Vinegar is an acetic acid solution in unsaturated water. Vinegar is an unsaturated solution of acetic. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. In. Example Of Unsaturated Solvent.
From www.youtube.com
Saturated and unsaturated solutions YouTube Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. Apply a solubility conversion factor to calculate the amount of solute that can be. Example Of Unsaturated Solvent.
From slideplayer.com
Saturated and Unsaturated Solutions ppt download Example Of Unsaturated Solvent Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Vinegar is an acetic acid solution in unsaturated water. Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. An unsaturated solution is a. Example Of Unsaturated Solvent.
From www.slideserve.com
PPT Liquid dosage forms PowerPoint Presentation ID4329709 Example Of Unsaturated Solvent Vinegar is an unsaturated solution of acetic. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. An unsaturated solution is a solution that contains less than the maximum. Example Of Unsaturated Solvent.
From www.vrogue.co
Unsaturated Hydrocarbon Alkenes Classnotes Ng vrogue.co Example Of Unsaturated Solvent Apply a solubility conversion factor to calculate the amount of solute that can be dissolved in a specified quantity of solvent. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. An unsaturated solution is a chemical solution in which the concentration of the solute is lower than the solubility of its equilibrium. In. Example Of Unsaturated Solvent.
From www.vedantu.com
Unsaturated Solutions Types and Examples of Unsaturated Solutions Example Of Unsaturated Solvent Vinegar is an acetic acid solution in unsaturated water. Adding a spoonful of sugar to a cup of hot coffee produces an unsaturated sugar solution. Unsaturated solutions are solutions in which the amount of dissolved solute is less than the saturation point of the solvent (at that specific temperature gradient). Apply a solubility conversion factor to calculate the amount of. Example Of Unsaturated Solvent.
From brainly.in
define unsaturated solution Brainly.in Example Of Unsaturated Solvent Vinegar is an unsaturated solution of acetic. In a specific gas mixture, the solute may be solid, liquid, or even gaseous, while gases act as the solvent. Any solution with a concentration of solute below the saturation point of the solute is an unsaturated solution. An unsaturated solution is a chemical solution in which the concentration of the solute is. Example Of Unsaturated Solvent.