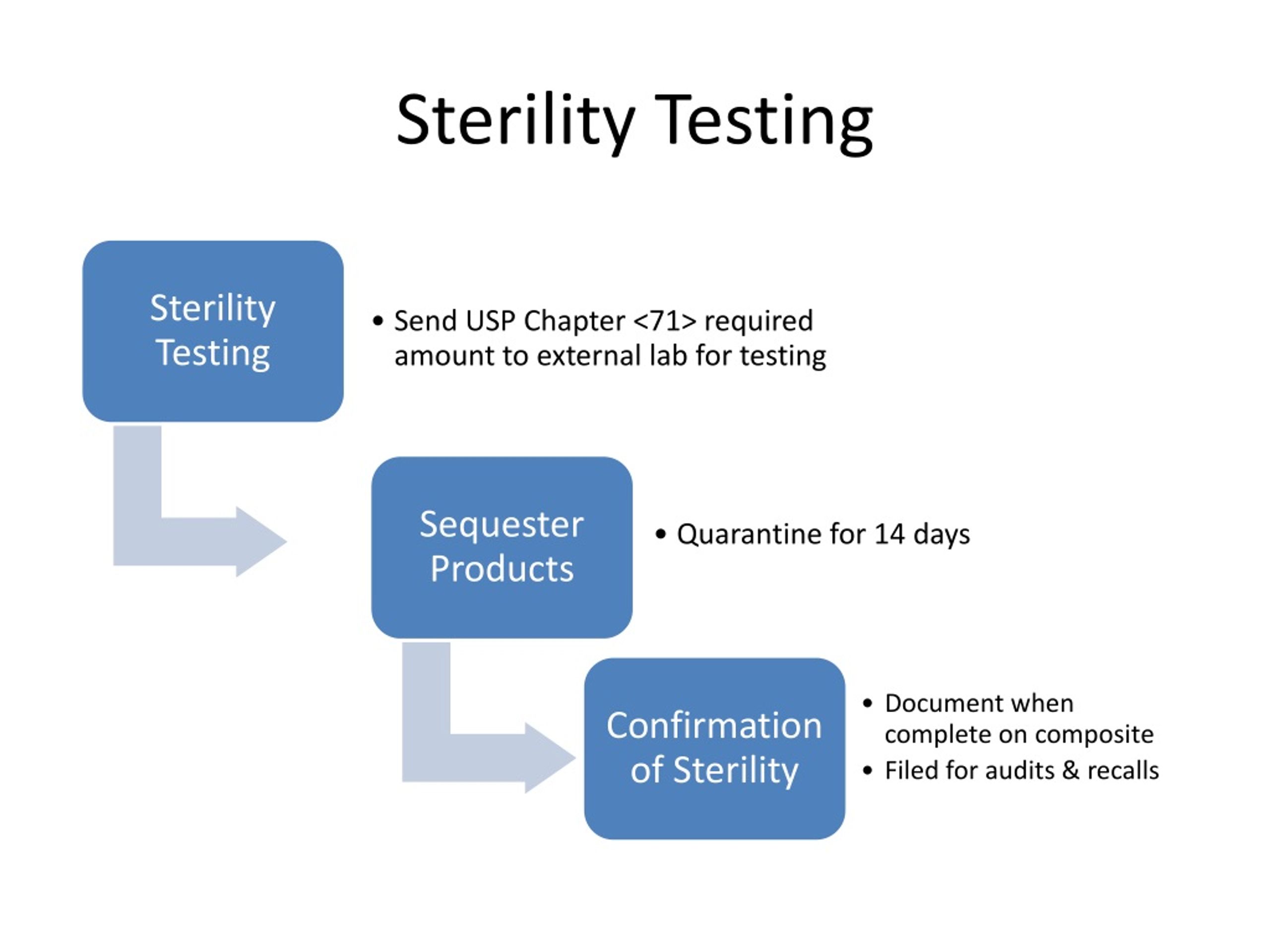
Method For Sterility Testing . Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Select from our range of sterility testing media. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Tests of sterility performed in the definition, validation. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. These products should pass sterility testing because unsterile.
from www.slideserve.com
Tests of sterility performed in the definition, validation. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. These products should pass sterility testing because unsterile. Select from our range of sterility testing media.
PPT Centralized Sterile Product Preparation Workflow for Anywhere Health System (AHS
Method For Sterility Testing This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Tests of sterility performed in the definition, validation. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. These products should pass sterility testing because unsterile. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology.
From www.youtube.com
How to perform sterility test for pharmaceutical sterile injectables YouTube Method For Sterility Testing Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. These products should pass sterility testing because unsterile. Tests of sterility performed in the definition, validation. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Sterility testing methods are diverse, tailored to specific product. Method For Sterility Testing.
From ceodhagq.blob.core.windows.net
Membrane Filtration Method Sterility Testing at Shera Cooks blog Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. These products should pass sterility testing because. Method For Sterility Testing.
From www.slideserve.com
PPT Centralized Sterile Product Preparation Workflow for Anywhere Health System (AHS Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Select from our range of sterility testing media. These products should. Method For Sterility Testing.
From worldcomplianceseminars.com
Microbiological Rapid Method Sterility Testing (RMT) of Short Dated Method For Sterility Testing These products should pass sterility testing because unsterile. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Select from our range of sterility testing media. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the product sterility testing. Method For Sterility Testing.
From microbe-investigations.com
USP 71 Sterility Testing Services Microbe Investigations Method For Sterility Testing These products should pass sterility testing because unsterile. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products. Method For Sterility Testing.
From www.slideshare.net
Sterility test Method For Sterility Testing Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. These products should pass sterility testing because unsterile. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Sterility testing verifies. Method For Sterility Testing.
From www.slideserve.com
PPT Sterility Testing PowerPoint Presentation, free download ID6352798 Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Select from our range of sterility testing media. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Sterility testing verifies. Method For Sterility Testing.
From www.pharmaguideline.co.uk
Sterility Test, how to perform Sterility Test in the best way? Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. These products should pass sterility testing because unsterile. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Select from our range of sterility testing media. Tests of sterility performed in the definition, validation.. Method For Sterility Testing.
From www.slideserve.com
PPT STERILITY TEST PowerPoint Presentation, free download ID1994580 Method For Sterility Testing Select from our range of sterility testing media. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Tests of sterility performed in the definition, validation. These products should pass sterility testing because unsterile. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility. Method For Sterility Testing.
From microbeonline.com
Sterility Testing of Pharmaceuticals • Microbe Online Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. These products should pass sterility testing because unsterile. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Select from our range of sterility testing media. Tests of sterility performed in the definition, validation. Sterility testing verifies. Method For Sterility Testing.
From www.youtube.com
Rapid Sterility Testing An Alternative Microbiological Method YouTube Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. These products should pass sterility testing because unsterile. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Select from our. Method For Sterility Testing.
From www.youtube.com
Sterility Testing Membrane Filtration & Direct Inoculation Method / L5 Unit3 Microbiology Method For Sterility Testing These products should pass sterility testing because unsterile. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products. Method For Sterility Testing.
From www.slideserve.com
PPT STERILITY TEST PowerPoint Presentation, free download ID1994580 Method For Sterility Testing Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. These products should pass. Method For Sterility Testing.
From ceodhagq.blob.core.windows.net
Membrane Filtration Method Sterility Testing at Shera Cooks blog Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Tests of sterility performed in the definition, validation. These products should pass sterility testing because unsterile. Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing of pharmaceutical or medical products helps. Method For Sterility Testing.
From www.slideshare.net
Sterility tests Method For Sterility Testing Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Select from our range of sterility testing media. These products should pass sterility testing because unsterile. Tests of sterility performed in the definition, validation. The. Method For Sterility Testing.
From www.slideserve.com
PPT Microbiological Control Tests PowerPoint Presentation ID1269178 Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Tests of sterility performed in the definition, validation. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from. Method For Sterility Testing.
From www.food-men.com
Food Commercial Sterility Testing Method and Analysis Method For Sterility Testing This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Sterility testing of pharmaceutical or. Method For Sterility Testing.
From siglaboratory.com
Sterility Test SIG LABORATORY Method For Sterility Testing Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Select from our range of sterility testing media. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Discover usp 71 sterility. Method For Sterility Testing.
From www.slideshare.net
Sterility test Method For Sterility Testing Tests of sterility performed in the definition, validation. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Select. Method For Sterility Testing.
From www.slideshare.net
Sterility testing Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Tests of sterility performed in the definition, validation. These products should pass sterility testing because unsterile. Sterility testing methods are diverse, tailored to specific product. Method For Sterility Testing.
From microbiologyguideritesh.com
SOP for Sterility Test by Open Method Microbiology Guidelines Method For Sterility Testing Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Tests of sterility performed in the definition, validation. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including. Method For Sterility Testing.
From www.slideserve.com
PPT PHT 434 PowerPoint Presentation, free download ID3329067 Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. These products should pass sterility testing because unsterile. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing of pharmaceutical. Method For Sterility Testing.
From ethidelabs.com
Ethide Laboratories Sterility Testing Methods For Regulatory Testing Method For Sterility Testing Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. These products should pass sterility testing because unsterile. Tests of sterility performed in the definition, validation. Select from our range of sterility testing media.. Method For Sterility Testing.
From www.slideshare.net
Sterility tests Method For Sterility Testing Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. These products should pass sterility testing because unsterile. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Sterility testing of pharmaceutical or medical products helps assess whether they are. Method For Sterility Testing.
From solutionpharmacy.in
Sterility Test in Microbiology Solution Parmacy Method For Sterility Testing Select from our range of sterility testing media. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. This. Method For Sterility Testing.
From ceodhagq.blob.core.windows.net
Membrane Filtration Method Sterility Testing at Shera Cooks blog Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Tests of sterility performed in the definition, validation. Select from our range of sterility testing media. These products should pass sterility testing because unsterile. Discover usp 71. Method For Sterility Testing.
From ethidelabs.com
Ethide Laboratories Sterility Testing Methods For Regulatory Testing Method For Sterility Testing Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Tests of sterility performed in the definition, validation. These products should pass sterility testing because unsterile. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Discover usp 71 sterility testing methods, including membrane. Method For Sterility Testing.
From www.youtube.com
STERILITY TESTING Random Sampling techniques, Diluting Fluids, Culture media, Methods, Result Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation,. Method For Sterility Testing.
From www.youtube.com
Sterility testing of pharmaceuticals sterility testing Part5, Unit3 Microbiology 3rd Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Select from our range of sterility testing media. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products,. Method For Sterility Testing.
From www.pharmasopworld.com
Sterility Test validation Method For Sterility Testing This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals. Method For Sterility Testing.
From cmdclabs.com
The Evolution of Sterility Testing From USP to Advanced Methods CMDC Labs Method For Sterility Testing Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. These products should pass sterility testing because unsterile. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. This minireview provides an overview of (i) the product sterility testing process, including a description of the compendial methods for. Tests of sterility. Method For Sterility Testing.
From www.hylabs.co.il
Sterility test products (and Endotoxin test) Hylabs Method For Sterility Testing Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. These products should pass sterility testing because unsterile. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. This minireview provides an overview of (i) the. Method For Sterility Testing.
From www.slideshare.net
Sterility tests Method For Sterility Testing Tests of sterility performed in the definition, validation. These products should pass sterility testing because unsterile. Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. The who sterility testing guidelines are applicable across. Method For Sterility Testing.
From www.tailingood.com
Sterility TestSolutionsZHEJIANG TAILIN BIOENGINEERING CO.,LTD Method For Sterility Testing Select from our range of sterility testing media. Sterility testing methods are diverse, tailored to specific product types and regulatory requirements. Discover usp 71 sterility testing methods, including membrane filtration and direct inoculation, to verify your products are free from harmful. These products should pass sterility testing because unsterile. This minireview provides an overview of (i) the product sterility testing. Method For Sterility Testing.
From www.slideserve.com
PPT Sterility Testing PowerPoint Presentation, free download ID6352798 Method For Sterility Testing The who sterility testing guidelines are applicable across a wide range of biological medicinal products including vaccines, blood products, biotechnology. Select from our range of sterility testing media. Sterility testing verifies the absence of viable contaminating microorganisms in sterile pharmaceuticals and medical devices. Sterility testing of pharmaceutical or medical products helps assess whether they are free from contaminating microorganisms. Tests. Method For Sterility Testing.