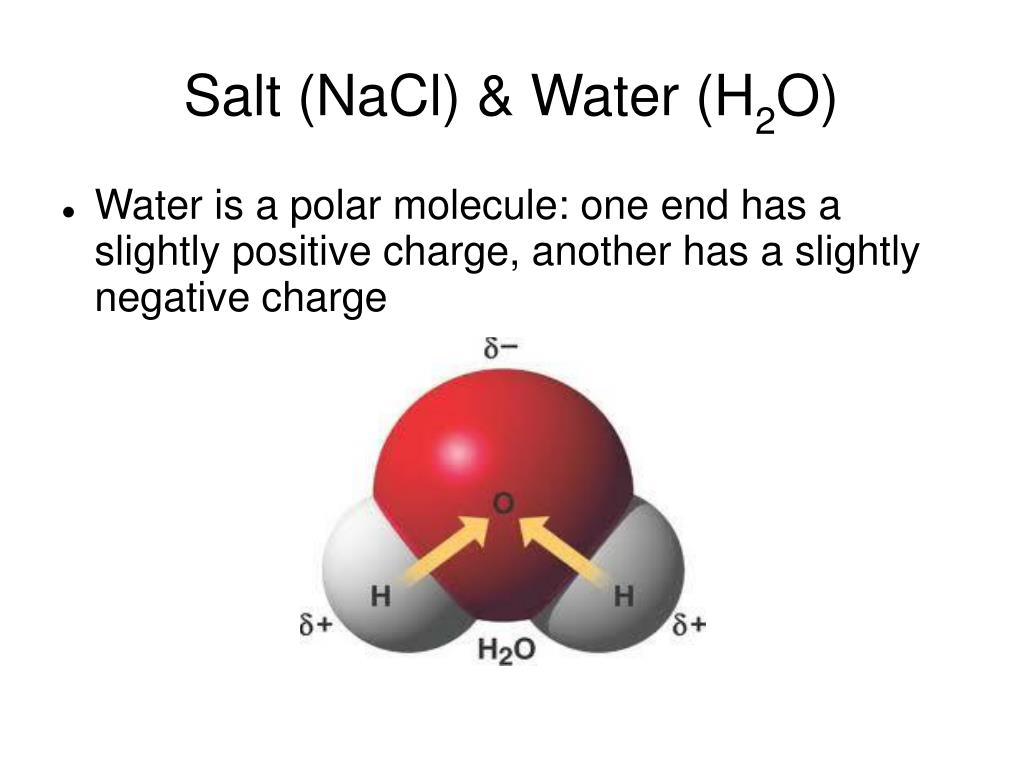
Why Does Water Dissolve Salt . When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Why salt dissolves in water. Water molecules pulling apart the ions (sodium and chloride) in a. The negative areas of the water molecules surround the positive sodium. Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them apart,. Nonpolar molecules such as those found in grease or oil do not dissolve in. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. The positive areas of the water molecules surround the negative chloride ions. A longstanding mystery about how salt dissolves in water has. This process is called hydration, and.
from www.slideserve.com
When salt is added to water, the positive and negative ions separate. Why salt dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. The positive areas of the water molecules surround the negative chloride ions. This process is called hydration, and. Nonpolar molecules such as those found in grease or oil do not dissolve in. The negative areas of the water molecules surround the positive sodium. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. A longstanding mystery about how salt dissolves in water has. The water molecules surround the ions and pull them apart,.
PPT Intermolecular Forces PowerPoint Presentation, free download ID4324596
Why Does Water Dissolve Salt The positive areas of the water molecules surround the negative chloride ions. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. The positive areas of the water molecules surround the negative chloride ions. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Nonpolar molecules such as those found in grease or oil do not dissolve in. A longstanding mystery about how salt dissolves in water has. When salt is added to water, the positive and negative ions separate. Why salt dissolves in water. The negative areas of the water molecules surround the positive sodium. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Water typically dissolves many ionic compounds and polar molecules. Water molecules pulling apart the ions (sodium and chloride) in a. The water molecules surround the ions and pull them apart,. This process is called hydration, and.
From www.slideserve.com
PPT SPONCH PowerPoint Presentation, free download ID556676 Why Does Water Dissolve Salt The water molecules surround the ions and pull them apart,. The negative areas of the water molecules surround the positive sodium. A longstanding mystery about how salt dissolves in water has. Nonpolar molecules such as those found in grease or oil do not dissolve in. When salt is added to water, the positive and negative ions separate. A machine learning. Why Does Water Dissolve Salt.
From butjustwhy.com
Why does salt dissolve in water? But Just Why!? Why Does Water Dissolve Salt Water typically dissolves many ionic compounds and polar molecules. A longstanding mystery about how salt dissolves in water has. Water molecules pulling apart the ions (sodium and chloride) in a. This process is called hydration, and. Why salt dissolves in water. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around. Why Does Water Dissolve Salt.
From studylib.net
Chapter 5, Lesson 3—Why Does Water Dissolve Salt? Why Does Water Dissolve Salt Why salt dissolves in water. The water molecules surround the ions and pull them apart,. Water molecules pulling apart the ions (sodium and chloride) in a. A longstanding mystery about how salt dissolves in water has. The positive areas of the water molecules surround the negative chloride ions. Water can dissolve salt because the positive part of water molecules attracts. Why Does Water Dissolve Salt.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Why Does Water Dissolve Salt At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Why salt dissolves in water. The water molecules surround the ions and pull them apart,. A longstanding mystery about how salt dissolves in water has. The negative areas of the water molecules surround the positive sodium. Water can dissolve salt because. Why Does Water Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Water Dissolve Salt A longstanding mystery about how salt dissolves in water has. Nonpolar molecules such as those found in grease or oil do not dissolve in. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Water typically dissolves many ionic compounds and polar molecules. The water molecules surround the ions and. Why Does Water Dissolve Salt.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Why Does Water Dissolve Salt Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water molecules pulling apart the ions (sodium and chloride) in a. Why salt dissolves in water. The positive areas of the water molecules surround the. Why Does Water Dissolve Salt.
From loeiulzbj.blob.core.windows.net
Why The Salt Dissolves In Water at Beverly Leach blog Why Does Water Dissolve Salt The water molecules surround the ions and pull them apart,. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water molecules pulling apart the ions (sodium and chloride) in a. This process is called hydration,. Why Does Water Dissolve Salt.
From www.acs.org
Lesson 5.3 Why Does Water Dissolve Salt? American Chemical Society Why Does Water Dissolve Salt Water molecules pulling apart the ions (sodium and chloride) in a. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. The water molecules surround the ions and pull them apart,. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both.. Why Does Water Dissolve Salt.
From www.youtube.com
Why Salt (NaCl) dissolve in water YouTube Why Does Water Dissolve Salt Water molecules pulling apart the ions (sodium and chloride) in a. Why salt dissolves in water. The negative areas of the water molecules surround the positive sodium. A longstanding mystery about how salt dissolves in water has. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. The positive. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID2736092 Why Does Water Dissolve Salt The negative areas of the water molecules surround the positive sodium. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. The positive areas of the water molecules surround the negative chloride ions. The water molecules surround the ions and pull them apart,. Why salt dissolves in water. A machine. Why Does Water Dissolve Salt.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Why Does Water Dissolve Salt The negative areas of the water molecules surround the positive sodium. Water typically dissolves many ionic compounds and polar molecules. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. A longstanding mystery about how salt dissolves in water has. When salt is added to water, the positive and. Why Does Water Dissolve Salt.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Library Koyuncu Salt Why Does Water Dissolve Salt Water typically dissolves many ionic compounds and polar molecules. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Why salt dissolves in water. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. The water molecules surround the. Why Does Water Dissolve Salt.
From exonfafiq.blob.core.windows.net
Does Salt Dissolve In Room Temperature Water at Peter Allen blog Why Does Water Dissolve Salt A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. The water molecules surround the ions and pull them apart,. Why salt dissolves in water. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A longstanding mystery about. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID6136171 Why Does Water Dissolve Salt The positive areas of the water molecules surround the negative chloride ions. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then. Why Does Water Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Water Dissolve Salt Why salt dissolves in water. Water molecules pulling apart the ions (sodium and chloride) in a. When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Water typically dissolves. Why Does Water Dissolve Salt.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Library Koyuncu Salt Why Does Water Dissolve Salt A longstanding mystery about how salt dissolves in water has. The positive areas of the water molecules surround the negative chloride ions. Nonpolar molecules such as those found in grease or oil do not dissolve in. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Why salt dissolves. Why Does Water Dissolve Salt.
From www.youtube.com
How Salt Dissolves in Water? YouTube Why Does Water Dissolve Salt Why salt dissolves in water. The positive areas of the water molecules surround the negative chloride ions. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. The negative areas of the water molecules surround the positive sodium. When salt is added to water, the positive and negative ions. Why Does Water Dissolve Salt.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Why Does Water Dissolve Salt When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. The positive areas of the water molecules surround the negative chloride ions. The water molecules surround the ions and pull them apart,. The negative areas of the water molecules surround the positive sodium. Nonpolar molecules such as those found in. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID3057625 Why Does Water Dissolve Salt The positive areas of the water molecules surround the negative chloride ions. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Why salt dissolves in water. Water molecules pulling. Why Does Water Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Water Dissolve Salt The negative areas of the water molecules surround the positive sodium. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. Why salt dissolves in water. At the. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID4324596 Why Does Water Dissolve Salt When salt is added to water, the positive and negative ions separate. This process is called hydration, and. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Water typically dissolves many ionic compounds and polar molecules. The negative areas of the water molecules surround the positive sodium. The. Why Does Water Dissolve Salt.
From mungfali.com
Dissolving Salt In Water Diagram Why Does Water Dissolve Salt The water molecules surround the ions and pull them apart,. Nonpolar molecules such as those found in grease or oil do not dissolve in. This process is called hydration, and. Why salt dissolves in water. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. When salt is added to water,. Why Does Water Dissolve Salt.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Does Water Dissolve Salt When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. Why salt dissolves in water. A longstanding mystery about how salt dissolves in water has. Nonpolar molecules such as those found. Why Does Water Dissolve Salt.
From www.youtube.com
Why Does Water Dissolve Salt? YouTube Why Does Water Dissolve Salt The negative areas of the water molecules surround the positive sodium. The water molecules surround the ions and pull them apart,. This process is called hydration, and. When salt is added to water, the positive and negative ions separate. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of.. Why Does Water Dissolve Salt.
From www.youtube.com
How does water dissolve salt? YouTube Why Does Water Dissolve Salt When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. This process is called hydration, and. Why salt dissolves in water. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Nonpolar molecules such as those found in grease. Why Does Water Dissolve Salt.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Why Does Water Dissolve Salt Nonpolar molecules such as those found in grease or oil do not dissolve in. Water typically dissolves many ionic compounds and polar molecules. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Why salt dissolves in water. When salt is added to water, the water molecules surround the. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free download ID1242210 Why Does Water Dissolve Salt When salt is added to water, the water molecules surround the ions and form a sphere of hydration around them. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Water typically dissolves many ionic compounds and polar molecules. At the molecular level, salt dissolves in water due to. Why Does Water Dissolve Salt.
From mungfali.com
Dissolving Salt In Water Diagram Why Does Water Dissolve Salt Water typically dissolves many ionic compounds and polar molecules. A longstanding mystery about how salt dissolves in water has. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part. Why Does Water Dissolve Salt.
From www.acs.org
Lesson 5.3 Why Does Water Dissolve Salt? American Chemical Society Why Does Water Dissolve Salt Why salt dissolves in water. The negative areas of the water molecules surround the positive sodium. A longstanding mystery about how salt dissolves in water has. This process is called hydration, and. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. When salt is added to water, the. Why Does Water Dissolve Salt.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Does Water Dissolve Salt A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. This process is called hydration, and. The water molecules surround the ions and pull them apart,. The negative areas of the water molecules surround the positive sodium. Water molecules pulling apart the ions (sodium and chloride) in a. The. Why Does Water Dissolve Salt.
From www.studypool.com
SOLUTION Why does water dissolve salt? Studypool Why Does Water Dissolve Salt This process is called hydration, and. Water typically dissolves many ionic compounds and polar molecules. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. The positive areas. Why Does Water Dissolve Salt.
From www.youtube.com
Why Does Water Dissolve Salt? YouTube Why Does Water Dissolve Salt The water molecules surround the ions and pull them apart,. Why salt dissolves in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Water molecules pulling apart the ions (sodium and chloride) in a. A machine learning model has revealed how crystals of sodium chloride slowly weaken. Why Does Water Dissolve Salt.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Does Water Dissolve Salt This process is called hydration, and. When salt is added to water, the positive and negative ions separate. At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both. A longstanding mystery about how salt dissolves in water has. Why salt dissolves in water. The water molecules surround the ions and pull. Why Does Water Dissolve Salt.
From slideplayer.com
Quarter Three Notes. ppt download Why Does Water Dissolve Salt Why salt dissolves in water. Water typically dissolves many ionic compounds and polar molecules. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water. Nonpolar molecules such as those found in grease or oil do not dissolve in. The negative areas of the water molecules surround the positive sodium.. Why Does Water Dissolve Salt.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Why Does Water Dissolve Salt Water typically dissolves many ionic compounds and polar molecules. This process is called hydration, and. The water molecules surround the ions and pull them apart,. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. When salt is added to water, the water molecules surround the ions and form. Why Does Water Dissolve Salt.