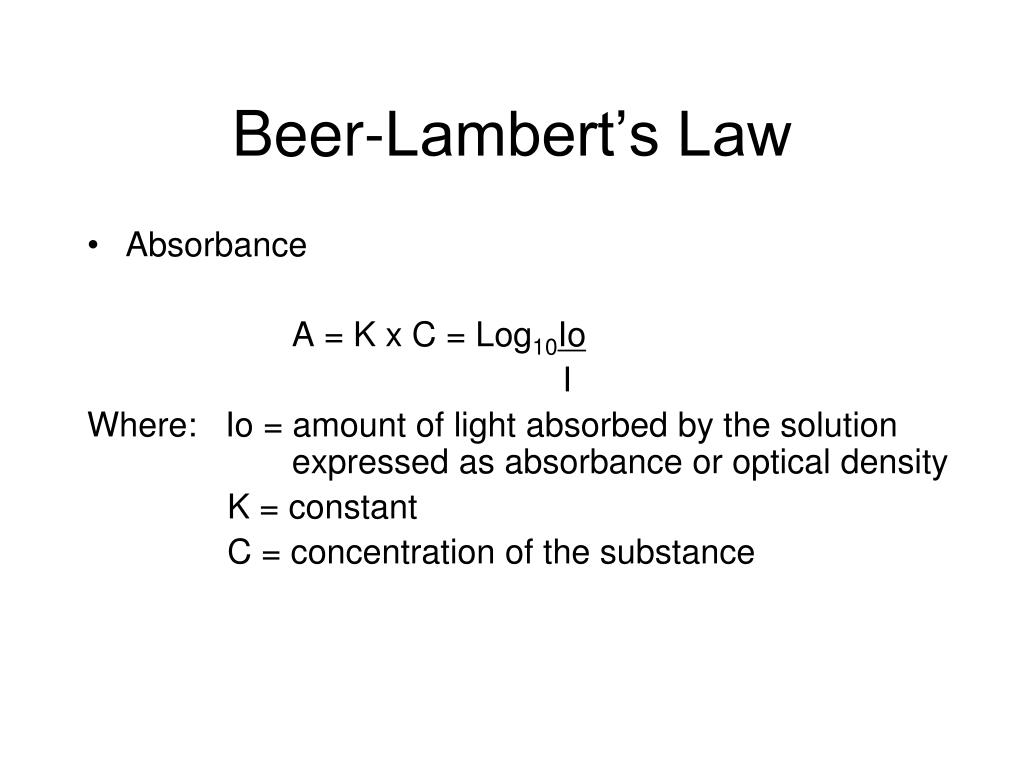
Beer Lambert Law High Concentration . In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
from www.slideserve.com
Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is.
PPT SPECTROPHOTOMETRY PowerPoint Presentation, free download ID5761859
Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.youtube.com
B.7 The Beer Lambert law (HL) YouTube Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From slideplayer.com
INSTRUMENTAL ANALYSIS ppt download Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From slidetodoc.com
Beers Lambert Law and Standard Curves of concentrations Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From studylib.net
Beer Lambert Law Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.chegg.com
Solved The BeerLambert Law is useful for determining the Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.slideserve.com
PPT SPECTROPHOTOMETRY PowerPoint Presentation, free download ID5761859 Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.youtube.com
Absorption spectroscopy (BeerLambert law) presented by PhD Emil Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.researchgate.net
3. Illustration to Beerlambert's law. Download Scientific Diagram Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From slidetodoc.com
Beers Lambert Law and Standard Curves of concentrations Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.studocu.com
Abreviated Report 2 Application of the BeerLambert Law to Track the Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.researchgate.net
1 BeerLambert law; Xrays to solid matter interaction. Download Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer Lambert Law High Concentration The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at. Beer Lambert Law High Concentration.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation ID2407366 Beer Lambert Law High Concentration In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law High Concentration.
From www.numerade.com
The BeerLambert Law is used in spectroscopy to determine the molar Beer Lambert Law High Concentration Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The law is simply an application of the observation that, within certain ranges, the absorbance of a chromophore at a given. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance. Beer Lambert Law High Concentration.