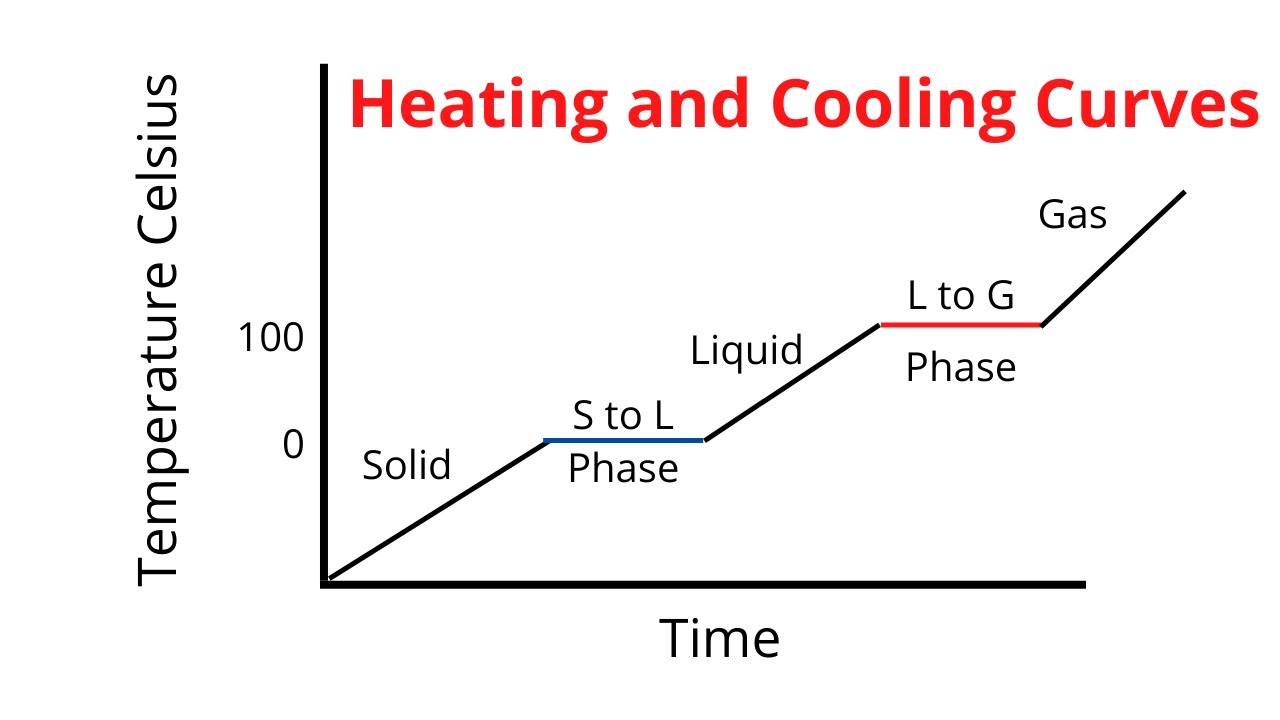
Heating Curve Answers . Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. What is the freezing point temperature of the substance? Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve of an unknown substance. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa).
from www.youtube.com
Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve of an unknown substance. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). What is the freezing point temperature of the substance? It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the.
Heating and Cooling Curve / Introduction plus and Potential
Heating Curve Answers Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. What is the freezing point temperature of the substance? A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. A heating curve of an unknown substance.
From wordworksheet.com
Heating And Cooling Curves Worksheet Heating Curve Answers What is the freezing point temperature of the substance? A heating curve of an unknown substance. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. A student heats 100 g of an unknown solid. Heating Curve Answers.
From zipworksheet.com
Heating Curve Worksheet Answers Heating Curve Answers It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. A heating curve of an unknown substance. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Base your answers to questions 54 and 55 on the heating curve below,. Heating Curve Answers.
From worksheets.decoomo.com
20++ Heating Curve Worksheet Answers Worksheets Decoomo Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Figure \(\pageindex{3}\) shows a heating curve, a. Heating Curve Answers.
From answermediabrandt.z19.web.core.windows.net
Heating Curve Worksheet With Answers Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. What is the freezing point temperature of the substance? A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid. Heating Curve Answers.
From www.onlineworksheet.my.id
Heating Curve Worksheet Answers Onlineworksheet.my.id Heating Curve Answers A heating curve of an unknown substance. What is the freezing point temperature of the substance? It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A student heats 100. Heating Curve Answers.
From www.onlineworksheet.my.id
Heating Curve Worksheet Answers Onlineworksheet.my.id Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. A heating curve of an unknown substance.. Heating Curve Answers.
From printablefulltim.z19.web.core.windows.net
Heating Cooling Curves Worksheet Answers Heating Curve Answers Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. Base your answers to questions. Heating Curve Answers.
From www.chegg.com
Solved 4/28/21 Period 6 Heating Curves Worksheet Answer Heating Curve Answers It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. What is the freezing point. Heating Curve Answers.
From answerlistjurgen.z19.web.core.windows.net
Heating Curve Worksheets With Answers Heating Curve Answers A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. What is the freezing point temperature of the substance? Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a. Heating Curve Answers.
From www.scribd.com
82218 Heating and Cooling Curve Answers PDF Heating Curve Answers Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point. Heating Curve Answers.
From materiallistgaskell.z21.web.core.windows.net
Heating And Cooling Curves Practice Heating Curve Answers A heating curve of an unknown substance. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid and gas. Heating Curve Answers.
From answermediabrandt.z19.web.core.windows.net
Heating Curve Worksheet Answers Heating Curve Answers It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. A heating curve of an unknown substance. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A student heats 100 g of an unknown solid substance at 1 atm. Heating Curve Answers.
From davida.davivienda.com
Heating Curve Worksheet With Answers Printable Word Searches Heating Curve Answers What is the freezing point temperature of the substance? Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. A heating curve of an unknown substance. Base your answers to questions 54 and 55 on. Heating Curve Answers.
From worksheets.decoomo.com
30++ Heating Curve Worksheet Answer Key Worksheets Decoomo Heating Curve Answers A heating curve of an unknown substance. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). It is likely that the. Heating Curve Answers.
From studylib.net
heating curve worksheet Heating Curve Answers Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. What is the freezing point temperature of the substance? Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve Answers.
From obropolox.blogspot.com
39 heating cooling curve calculations worksheet answers Worksheet Heating Curve Answers What is the freezing point temperature of the substance? A heating curve of an unknown substance. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Identifying solid, liquid and. Heating Curve Answers.
From studyfinder.org
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling Heating Curve Answers What is the freezing point temperature of the substance? Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. It is likely that the heat of vaporization will have a larger magnitude since in the. Heating Curve Answers.
From www.scribd.com
1d Heating and Cooling Curves ANSWERS PDF Heating Curve Answers Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Identifying solid, liquid and gas phases, graph. Heating Curve Answers.
From gersgiasbwa.blogspot.com
41 heating curve worksheet answers key Worksheet Master Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). It is likely that the heat of vaporization will have a larger. Heating Curve Answers.
From materialdbhutchins.z21.web.core.windows.net
Heating Curve Of Water Answers Heating Curve Answers A heating curve of an unknown substance. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. It is likely. Heating Curve Answers.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Answers Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. It is likely that the heat of. Heating Curve Answers.
From www.onlineworksheet.my.id
Heating Curve Worksheet Answers Onlineworksheet.my.id Heating Curve Answers It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve of an unknown substance. What is the freezing point temperature of the substance? A student heats 100. Heating Curve Answers.
From goodimg.co
️A Heating Curve Worksheet Answers Free Download Goodimg.co Heating Curve Answers What is the freezing point temperature of the substance? A heating curve of an unknown substance. Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. It is likely that the heat of vaporization will. Heating Curve Answers.
From worksheetlistmo.z21.web.core.windows.net
Heating Curve Of Water Worksheets Answers Heating Curve Answers A heating curve of an unknown substance. What is the freezing point temperature of the substance? A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade,. Heating Curve Answers.
From learningschoolandy.z21.web.core.windows.net
Heating Curve Of Water Answers Heating Curve Answers A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. What is the freezing point temperature of the substance? A heating curve. Heating Curve Answers.
From studyfullschmid.z19.web.core.windows.net
Heating Curve Worksheet Answer Key Heating Curve Answers A heating curve of an unknown substance. What is the freezing point temperature of the substance? A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Base your answers to questions 54 and 55 on. Heating Curve Answers.
From zipworksheet.com
Heating Curve Worksheet Answers Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve of an unknown substance.. Heating Curve Answers.
From www.onlineworksheet.my.id
Heating Curve Worksheet Answers Onlineworksheet.my.id Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A student heats 100 g of an. Heating Curve Answers.
From www.chegg.com
Solved For Review 432e 98. Use the heatingcooling curve Heating Curve Answers Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. What is the freezing point temperature of the substance? Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating Curve Answers.
From zipworksheet.com
Heating Curve Worksheet Answers Heating Curve Answers Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. A heating curve of an. Heating Curve Answers.
From lessonmagicnoble99.z19.web.core.windows.net
Heating Cooling Curve Worksheets Answers Heating Curve Answers Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. It is likely that the heat of. Heating Curve Answers.
From www.studocu.com
Heating Cooling Curve Worksheet Studocu Heating Curve Answers What is the freezing point temperature of the substance? A heating curve of an unknown substance. A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade,. Heating Curve Answers.
From brainly.com
Examine the heating curve for water below. Answer each question Heating Curve Answers A student heats 100 g of an unknown solid substance at 1 atm (101 kpa). Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point and being heated at a constant rate over. It is likely that the heat of vaporization will have a larger. Heating Curve Answers.
From worksheetdbtrommler.z19.web.core.windows.net
Heating And Cooling Curves Worksheet Answers Heating Curve Answers A heating curve of an unknown substance. It is likely that the heat of vaporization will have a larger magnitude since in the case of vaporization the. Figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Base your answers to questions 54 and 55 on the heating curve below,. Heating Curve Answers.
From obropolox.blogspot.com
43 heating cooling curve worksheet answers Worksheet Resource Heating Curve Answers Identifying solid, liquid and gas phases, graph to show the melting and boiling point of a liquid, a series of free science lessons for 7th grade and 8th grade, ks3 and checkpoint, gcse and igcse. Base your answers to questions 54 and 55 on the heating curve below, which represents a substance starting as a solid below its melting point. Heating Curve Answers.