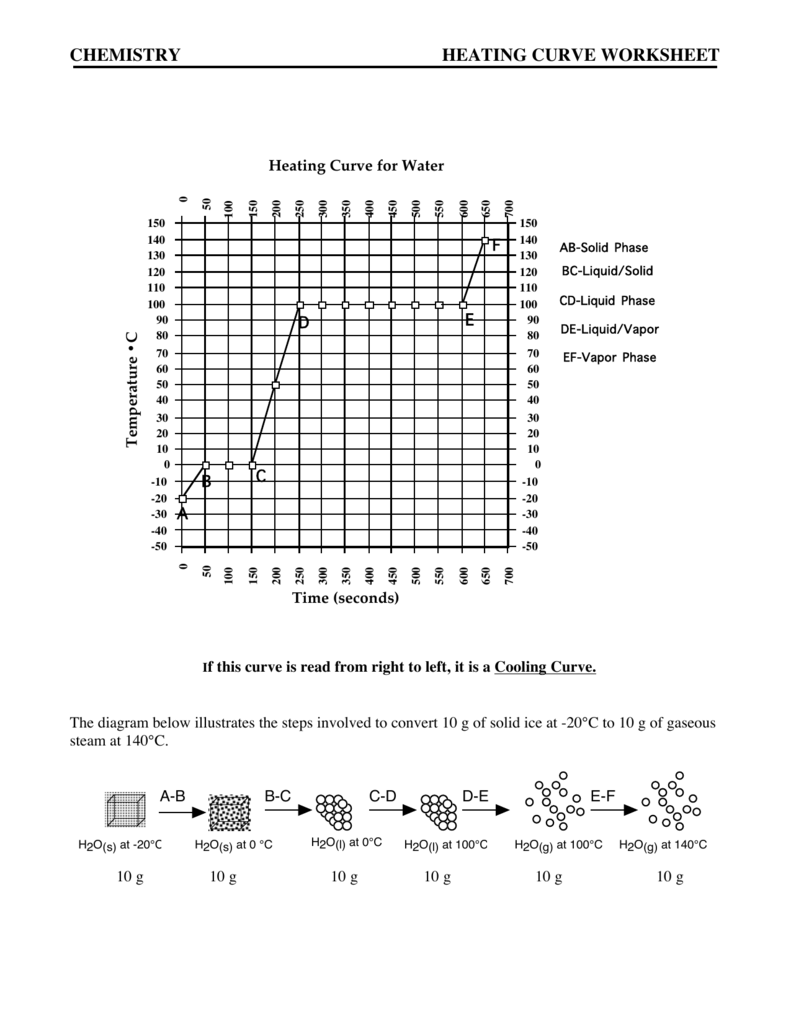
Heating Curve Calculations Answer Key . Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. On the phase diagram, label the gas and liquid regions. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the boiling point temperature of the substance? The general equation for calculating heat energy to change the temperature of a solid is: Answer the following using the above heating curve. A) which phases and transitions are required? Heat = mass x specific heat (solid) x temperature change q = m c. What is the melting point temperature of the substance? What is the melting temperature of the above substance?
from herbalens.blogspot.com
What is the melting point temperature of the substance? Answer the following using the above heating curve. On the phase diagram, label the gas and liquid regions. Heat = mass x specific heat (solid) x temperature change q = m c. What is the melting temperature of the above substance? The general equation for calculating heat energy to change the temperature of a solid is: Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the boiling point temperature of the substance? A) which phases and transitions are required? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is.
Heating Curve Worksheet Answer Key Herbalens
Heating Curve Calculations Answer Key On the phase diagram, label the gas and liquid regions. What is the melting temperature of the above substance? The general equation for calculating heat energy to change the temperature of a solid is: Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. Heat = mass x specific heat (solid) x temperature change q = m c. A) which phases and transitions are required? On the phase diagram, label the gas and liquid regions. What is the melting point temperature of the substance? Answer the following using the above heating curve. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the boiling point temperature of the substance?
From worksheets.decoomo.com
30++ Heating Curve Worksheet Answer Key Worksheets Decoomo Heating Curve Calculations Answer Key Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. Heat = mass x specific heat (solid) x temperature change q = m c. Answer the following using the above heating curve. A) which phases and transitions are required? On the phase diagram, label the gas. Heating Curve Calculations Answer Key.
From www.e-streetlight.com
Heating And Cooling Curve Worksheet E Street Light Heating Curve Calculations Answer Key Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the melting temperature of the above substance? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy. Heating Curve Calculations Answer Key.
From heatinggondon.blogspot.com
Heating Heating Curve Worksheet Heating Curve Calculations Answer Key On the phase diagram, label the gas and liquid regions. What is the boiling point temperature of the substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Answer the following using the above heating curve. What is the melting temperature of the above substance? What is the melting. Heating Curve Calculations Answer Key.
From studytofux1066t.z21.web.core.windows.net
Heating Curve Calculations Worksheets Heating Curve Calculations Answer Key Answer the following using the above heating curve. The general equation for calculating heat energy to change the temperature of a solid is: What is the melting point temperature of the substance? Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools.. Heating Curve Calculations Answer Key.
From www.chegg.com
Solved 4/28/21 Period 6 Heating Curves Worksheet Answer Heating Curve Calculations Answer Key Answer the following using the above heating curve. The general equation for calculating heat energy to change the temperature of a solid is: What is the melting temperature of the above substance? What is the boiling point temperature of the substance? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or. Heating Curve Calculations Answer Key.
From www.worksheetsgo.com
Heating Curve Worksheets WorksheetsGO Heating Curve Calculations Answer Key What is the melting point temperature of the substance? What is the boiling point temperature of the substance? What is the melting temperature of the above substance? The general equation for calculating heat energy to change the temperature of a solid is: On the phase diagram, label the gas and liquid regions. Heat = mass x specific heat (solid) x. Heating Curve Calculations Answer Key.
From chessmuseum.org
50 Heating Curve Worksheet Answers Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The general equation for calculating heat energy to change the temperature of a solid is: What is the melting temperature of the above substance? Heat = mass x specific heat (solid) x temperature change q = m c. Heating curve. Heating Curve Calculations Answer Key.
From www.scribd.com
Heating and Cooling Curve Calculations v2 PDF Calorie Heating Curve Calculations Answer Key Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the boiling point temperature of the substance? The general equation for calculating heat energy to change the temperature of a solid is: Answer the following using the above heating curve. What is the melting. Heating Curve Calculations Answer Key.
From worksheetzone.org
Heating Curve Worksheet Worksheet Heating Curve Calculations Answer Key What is the boiling point temperature of the substance? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the melting point temperature of the substance? Answer the following using the above heating curve. Heat = mass x specific heat (solid) x temperature change. Heating Curve Calculations Answer Key.
From obropolox.blogspot.com
39 heating cooling curve calculations worksheet answers Worksheet Heating Curve Calculations Answer Key Heat = mass x specific heat (solid) x temperature change q = m c. A) which phases and transitions are required? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that. Heating Curve Calculations Answer Key.
From www.youtube.com
Heating Curve Calculations YouTube Heating Curve Calculations Answer Key Answer the following using the above heating curve. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. The general equation for calculating heat energy to. Heating Curve Calculations Answer Key.
From worksheets.decoomo.com
30++ Heating Curve Worksheet Answer Key Worksheets Decoomo Heating Curve Calculations Answer Key Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the melting point temperature of the. Heating Curve Calculations Answer Key.
From studylistgerste.z19.web.core.windows.net
Heating Curve Worksheets Answer Key Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: What is the melting temperature of the above substance? Answer the following using the above heating curve. Heat = mass x specific heat (solid) x temperature change q = m c. On the phase diagram, label the gas and liquid. Heating Curve Calculations Answer Key.
From gersgiasbwa.blogspot.com
41 heating curve worksheet answers key Worksheet Master Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: A) which phases and transitions are required? Answer the following using the above heating curve. On the phase diagram, label the gas and liquid regions. What is the melting point temperature of the substance? Heat = mass x specific heat. Heating Curve Calculations Answer Key.
From worksheets.decoomo.com
20++ Heat Calculations Worksheet Answers Worksheets Decoomo Heating Curve Calculations Answer Key Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the melting temperature of the above substance? The general equation for calculating heat energy to change the temperature of a solid is: On the phase diagram, label the gas and liquid regions. Answer the. Heating Curve Calculations Answer Key.
From goodimg.co
️A Heating Curve Worksheet Answers Free Download Goodimg.co Heating Curve Calculations Answer Key What is the melting point temperature of the substance? Answer the following using the above heating curve. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The general equation for calculating heat energy to change the temperature of a solid is: What is the boiling point temperature of the. Heating Curve Calculations Answer Key.
From studytofux1066t.z21.web.core.windows.net
Heating Curve Calculations Worksheets Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Answer the following using the above heating curve. A) which phases and transitions are required? Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance. Heating Curve Calculations Answer Key.
From herbalens.blogspot.com
Heating Curve Worksheet Answer Key Herbalens Heating Curve Calculations Answer Key Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. On the phase diagram, label the gas and liquid regions. Answer the following using the above heating curve. The general equation for calculating heat energy to change the temperature of a solid is: What is the. Heating Curve Calculations Answer Key.
From www.chegg.com
Solved Heating Curve Calculations Worksheet Use the data for Heating Curve Calculations Answer Key The general equation for calculating heat energy to change the temperature of a solid is: On the phase diagram, label the gas and liquid regions. What is the boiling point temperature of the substance? Answer the following using the above heating curve. Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts. Heating Curve Calculations Answer Key.
From www.youtube.com
Heating Curve Calculation YouTube Heating Curve Calculations Answer Key Heat = mass x specific heat (solid) x temperature change q = m c. The general equation for calculating heat energy to change the temperature of a solid is: Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. Heating curves show that energy is absorbed. Heating Curve Calculations Answer Key.
From studylib.net
Heating Curve Worksheet (1) Heating Curve Calculations Answer Key The general equation for calculating heat energy to change the temperature of a solid is: Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. Answer. Heating Curve Calculations Answer Key.
From lessonstone.z13.web.core.windows.net
Heating Curve Worksheet 1 Heating Curve Calculations Answer Key A) which phases and transitions are required? On the phase diagram, label the gas and liquid regions. What is the boiling point temperature of the substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The general equation for calculating heat energy to change the temperature of a solid. Heating Curve Calculations Answer Key.
From answermediabrandt.z19.web.core.windows.net
Heating Curve Worksheet With Answers Heating Curve Calculations Answer Key Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the melting point temperature of the substance? A) which phases and transitions are required? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms. Heating Curve Calculations Answer Key.
From www.scribd.com
1d Heating and Cooling Curves ANSWERS PDF Heating Curve Calculations Answer Key What is the melting point temperature of the substance? Answer the following using the above heating curve. The general equation for calculating heat energy to change the temperature of a solid is: Heat = mass x specific heat (solid) x temperature change q = m c. What is the melting temperature of the above substance? Elemental carbon has one gas. Heating Curve Calculations Answer Key.
From www.youtube.com
Heating curve calculation (benzene) YouTube Heating Curve Calculations Answer Key Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. A) which phases and transitions are required? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What. Heating Curve Calculations Answer Key.
From davida.davivienda.com
Heating Curve Worksheet With Answers Printable Word Searches Heating Curve Calculations Answer Key Answer the following using the above heating curve. What is the melting point temperature of the substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: What is the boiling point temperature of the substance? The general equation for calculating heat energy to change the temperature of a solid. Heating Curve Calculations Answer Key.
From obropolox.blogspot.com
43 heating cooling curve worksheet answers Worksheet Resource Heating Curve Calculations Answer Key The general equation for calculating heat energy to change the temperature of a solid is: Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. A) which phases and transitions are required? On the phase diagram, label the gas and liquid regions.. Heating Curve Calculations Answer Key.
From www.studypool.com
SOLUTION Heatingcurveworksheet key Studypool Heating Curve Calculations Answer Key A) which phases and transitions are required? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the melting temperature of the above substance? Heat = mass x specific heat (solid) x temperature change q = m c. The general equation for calculating heat. Heating Curve Calculations Answer Key.
From novenalunasolitaria.blogspot.com
Heating Cooling Curve Worksheet Answer Key worksheet Heating Curve Calculations Answer Key Heat = mass x specific heat (solid) x temperature change q = m c. What is the melting temperature of the above substance? A) which phases and transitions are required? The general equation for calculating heat energy to change the temperature of a solid is: Heating curve worksheet heating curves show that energy is absorbed by a substance as it. Heating Curve Calculations Answer Key.
From www.chegg.com
Solved Per Name Heating Curve Calculations Go to the Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heat = mass x specific heat (solid) x temperature change q = m c. What is the boiling point temperature of the substance? On the phase diagram, label the gas and liquid regions. The general equation for calculating heat energy. Heating Curve Calculations Answer Key.
From www.scribd.com
Heating Curve of Water Worksheet Phase (Matter) Heat Heating Curve Calculations Answer Key A) which phases and transitions are required? What is the melting temperature of the above substance? Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. What is the boiling point temperature of the substance? On the phase diagram, label the gas and liquid regions. Heat. Heating Curve Calculations Answer Key.
From www.studocu.com
Heating Cooling Curve Worksheet Studocu Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: On the phase diagram, label the gas and liquid regions. What is the melting point temperature of the substance? Heat = mass x specific heat (solid) x temperature change q = m c. The general equation for calculating heat energy. Heating Curve Calculations Answer Key.
From herbalens.blogspot.com
Heating Curve Worksheet Answer Key Herbalens Heating Curve Calculations Answer Key A) which phases and transitions are required? What is the melting temperature of the above substance? What is the boiling point temperature of the substance? The general equation for calculating heat energy to change the temperature of a solid is: Answer the following using the above heating curve. Heating curve worksheet heating curves show that energy is absorbed by a. Heating Curve Calculations Answer Key.
From herbalens.blogspot.com
Heating Curve Worksheet Answer Key Herbalens Heating Curve Calculations Answer Key Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: What is the melting point temperature of the substance? What is the melting temperature of the above substance? A) which phases and transitions are required? Heat = mass x specific heat (solid) x temperature change q = m c. Heating. Heating Curve Calculations Answer Key.
From studyfinder.org
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling Heating Curve Calculations Answer Key On the phase diagram, label the gas and liquid regions. Heating curve worksheet heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as. Heating Curve Calculations Answer Key.