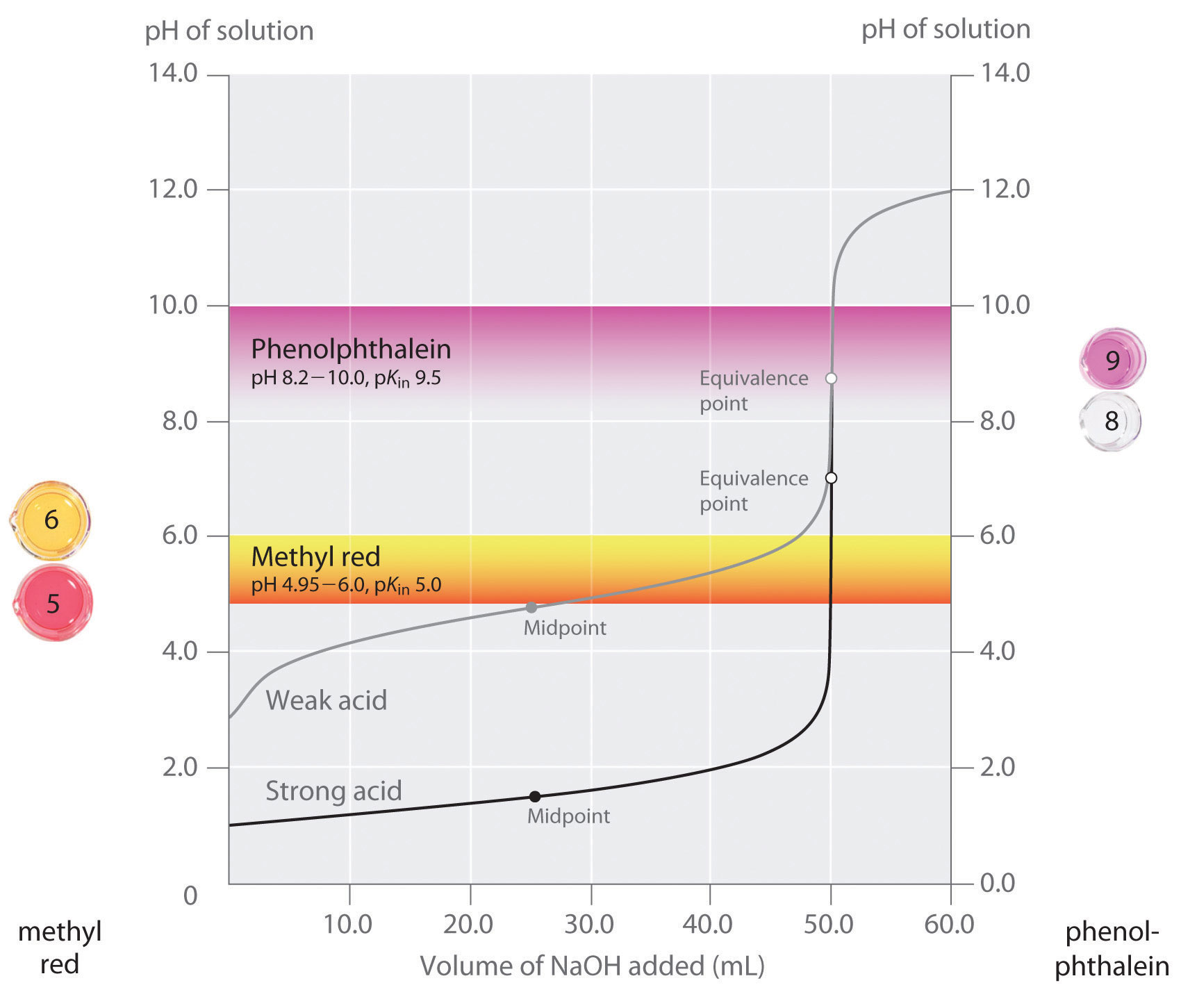
Which Indicator Is Used In This Titration . This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange.
from saylordotorg.github.io
Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances whose solutions change color due to changes in ph. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. This page assumes that you know about ph curves for all the.
AcidBase Titrations
Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a specific ph range. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Weak bases should be titrated in. Which Indicator Is Used In This Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Which Indicator Is Used In This Titration Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The graph shows the results obtained using two. Which Indicator Is Used In This Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Which Indicator Is Used In This Titration This page assumes that you know about ph curves for all the. Indicators are substances whose solutions change color due to changes in ph. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are. Which Indicator Is Used In This Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. This page assumes that you know about ph curves for all the. The two most common indicators. Which Indicator Is Used In This Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves. Which Indicator Is Used In This Titration.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Which Indicator Is Used In This Titration Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Both indicators change colour over a specific ph range. This page assumes that. Which Indicator Is Used In This Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Which Indicator Is Used In This Titration Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Both indicators change colour over. Which Indicator Is Used In This Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Which Indicator Is Used In This Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. Both. Which Indicator Is Used In This Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Which Indicator Is Used In This Titration Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using. Which Indicator Is Used In This Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Which Indicator Is Used In This Titration This page assumes that you know about ph curves for all the. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators. Which Indicator Is Used In This Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Indicators are substances whose solutions change color due to changes in ph. This page assumes that you know about ph curves. Which Indicator Is Used In This Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change colour over a specific ph range. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that. Which Indicator Is Used In This Titration.
From www.numerade.com
SOLVED Using the table of indicators identify which of the given Which Indicator Is Used In This Titration Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that. Which Indicator Is Used In This Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Which Indicator Is Used In This Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Both indicators change. Which Indicator Is Used In This Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak bases should be. Which Indicator Is Used In This Titration.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Which Indicator Is Used In This Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Both indicators change colour over a specific ph range. Indicators are substances whose solutions change color due to changes in ph. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions.. Which Indicator Is Used In This Titration.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the. Some commonly used indicators include bromothymol. Which Indicator Is Used In This Titration.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Which Indicator Is Used In This Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph.. Which Indicator Is Used In This Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra.. Which Indicator Is Used In This Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Which Indicator Is Used In This Titration Both indicators change colour over a specific ph range. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Indicators are substances whose solutions change color due to changes in ph.. Which Indicator Is Used In This Titration.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions.. Which Indicator Is Used In This Titration.
From www.toppr.com
The range of suitable indicator which should be used titration of NaX Which Indicator Is Used In This Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. This page assumes that you know about ph curves for all the.. Which Indicator Is Used In This Titration.
From www.studypool.com
SOLUTION Indicators used in titration Studypool Which Indicator Is Used In This Titration Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Indicators are substances whose solutions change color due to changes in ph. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange.. Which Indicator Is Used In This Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Which Indicator Is Used In This Titration Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Weak bases should be titrated in the. Which Indicator Is Used In This Titration.
From brainly.com
The equipment shown below is used for titrations. Name the piece of Which Indicator Is Used In This Titration Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. Weak bases should be. Which Indicator Is Used In This Titration.
From www.vrogue.co
Acid Base Titration With Phenolphthalein Indicator Wo vrogue.co Which Indicator Is Used In This Titration This page assumes that you know about ph curves for all the. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. Indicators are. Which Indicator Is Used In This Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Both indicators change. Which Indicator Is Used In This Titration.
From www.slideshare.net
Acid base titration Which Indicator Is Used In This Titration Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Both indicators change colour over a. Which Indicator Is Used In This Titration.
From mungfali.com
Acid Base Titration Indicator Which Indicator Is Used In This Titration Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. They are usually weak acids or bases,. Which Indicator Is Used In This Titration.
From www.science-revision.co.uk
Titrations Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. This page assumes that you know about ph curves for all the. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. Indicators are substances whose solutions change color due to changes in ph. Weak bases should be titrated in. Which Indicator Is Used In This Titration.
From saylordotorg.github.io
AcidBase Titrations Which Indicator Is Used In This Titration The two most common indicators that are used in titrations are methyl orange and phenolphthalein. Weak bases should be titrated in the presence of indicators which change under slightly acidic conditions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. Indicators are. Which Indicator Is Used In This Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Which Indicator Is Used In This Titration Indicators are substances whose solutions change color due to changes in ph. Some commonly used indicators include bromothymol blue, phenolphthalein, bromocresol green, methyl red, thymol blue, and methyl orange. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Weak. Which Indicator Is Used In This Titration.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Which Indicator Is Used In This Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Both indicators change colour over a specific ph range. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The graph shows the results obtained using two indicators (methyl red and. Which Indicator Is Used In This Titration.
From ar.inspiredpencil.com
Titration Setup Diagram Which Indicator Is Used In This Titration They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. The two most common indicators that are used in titrations are methyl orange and phenolphthalein. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. Indicators are substances whose solutions change. Which Indicator Is Used In This Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Which Indicator Is Used In This Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. This page assumes that you know about ph curves for all the. Both indicators change colour over a specific ph range. Weak acids are titrated in the presence of indicators which change under slightly alkaline conditions. The two most common indicators that are used in. Which Indicator Is Used In This Titration.