Regulatory Affairs Medical Devices . Reuter, kay taylor & george r. — david g. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. — regulatory affairs professionals serve a critical function throughout a medical device’s product. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically.
from md.sunflare.com
the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. — david g. — regulatory affairs professionals serve a critical function throughout a medical device’s product. Reuter, kay taylor & george r. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs.
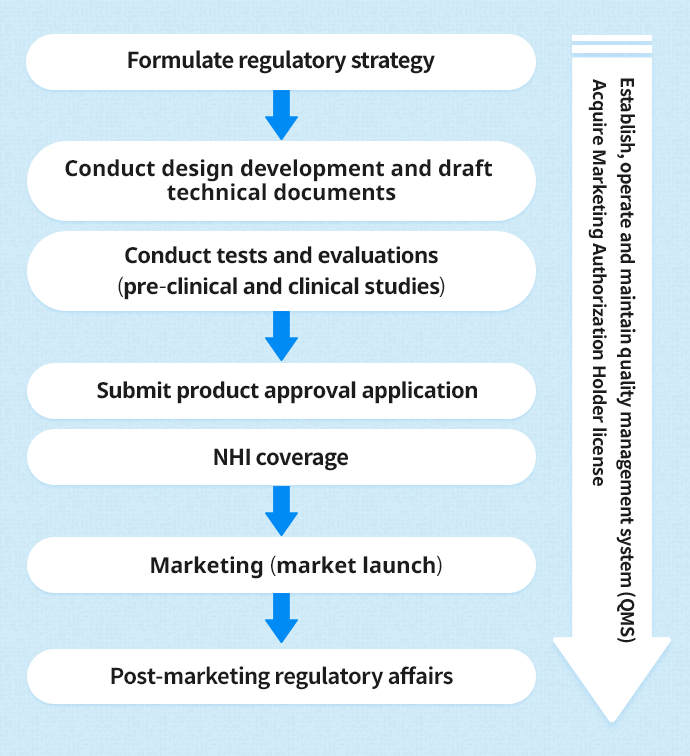
Regulatory affairs in Japan Total support for medical devices
Regulatory Affairs Medical Devices source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. — regulatory affairs professionals serve a critical function throughout a medical device’s product. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — david g. Reuter, kay taylor & george r. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been.
From www.insightaceanalytic.com
Medical Device Regulatory Affairs Market Size, Scope and Demand Analysis Regulatory Affairs Medical Devices the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. Reuter, kay. Regulatory Affairs Medical Devices.
From mdphysicianmag.com
Medical Device Regulatory Affairs Training Courses Lead by Industry Regulatory Affairs Medical Devices — david g. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device. Regulatory Affairs Medical Devices.
From www.youtube.com
Medical Device Quality Assurance and Regulatory Affairs StarFish Regulatory Affairs Medical Devices — regulatory affairs professionals serve a critical function throughout a medical device’s product. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. the level 8 higher diploma. Regulatory Affairs Medical Devices.
From medicalqms.com.au
Services • Medical Devices QMS and Regulatory Affairs Melbourne Regulatory Affairs Medical Devices The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. this msc provides. Regulatory Affairs Medical Devices.
From medicalqms.com.au
News and Updates • Medical Devices QMS and Regulatory Affairs Melbourne Regulatory Affairs Medical Devices Reuter, kay taylor & george r. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — david g. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. — regulatory affairs professionals serve a critical function throughout a medical device’s. Regulatory Affairs Medical Devices.
From medicalqms.com.au
Regulatory and QMS Training • Medical Devices QMS and Regulatory Regulatory Affairs Medical Devices — regulatory affairs professionals serve a critical function throughout a medical device’s product. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. source and interpret medical device directives currently regulating medical device. Regulatory Affairs Medical Devices.
From www.pharmatutor.org
Regulatory Affairs, Medical Devices Jobs at Pfizer PharmaTutor Regulatory Affairs Medical Devices The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. — david g. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. — regulatory affairs professionals serve a critical function throughout a medical device’s product. the. Regulatory Affairs Medical Devices.
From www.hardianhealth.com
Medical Device Regulatory Services UKCA, CE and FDA — Hardian Health Regulatory Affairs Medical Devices the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. Reuter, kay taylor & george r. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. — regulatory affairs professionals serve a critical function throughout a medical device’s product. — david g. . Regulatory Affairs Medical Devices.
From www.cyient.com
Whitepaper Overview of Medical Device Regulatory Affairs Regulatory Affairs Medical Devices Reuter, kay taylor & george r. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. source and interpret medical device directives currently regulating medical device classification within the. Regulatory Affairs Medical Devices.
From www.youtube.com
Global Medical Device Registration Impact of Changes to the EU MDR and Regulatory Affairs Medical Devices the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. the. Regulatory Affairs Medical Devices.
From www.linkedin.com
Excellent Job opportunity with leading Medical Device company as a Sr Regulatory Affairs Medical Devices source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. Reuter, kay taylor & george r. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. the level 8 certificate in medical technology regulatory affairs and quality. Regulatory Affairs Medical Devices.
From www.slideshare.net
Medical Device Regulatory Affairs. Regulatory Affairs Medical Devices Reuter, kay taylor & george r. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed. Regulatory Affairs Medical Devices.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Regulatory Affairs Medical Devices — david g. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. — regulatory affairs professionals serve a critical function throughout a medical device’s product. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. the level 8 certificate. Regulatory Affairs Medical Devices.
From royed.in
Medical Device Regulatory Affairs [PG Certification] Royed Training Regulatory Affairs Medical Devices the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. Reuter, kay taylor &. Regulatory Affairs Medical Devices.
From www.pg-medecon.de
Consulting for Medical Devices Peter Grübling Medical Device Consulting Regulatory Affairs Medical Devices the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — regulatory affairs professionals serve a critical function throughout a medical device’s product. — david g. Reuter, kay taylor & george r. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. source. Regulatory Affairs Medical Devices.
From www.rootsanalysis.com
Contract Regulatory Affairs Management Market for Medical Devices, 2019 Regulatory Affairs Medical Devices — regulatory affairs professionals serve a critical function throughout a medical device’s product. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. The level 8 certificate in medical technologies. Regulatory Affairs Medical Devices.
From zivadra.com
Demystifying Regulatory Affairs in Medical Devices A Comprehensive Regulatory Affairs Medical Devices The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. — david g. Reuter, kay taylor & george r. this msc provides graduates with an advanced theoretical understanding of the processes. Regulatory Affairs Medical Devices.
From www.mcra.com
Medical Device FDA Regulatory Consulting MCRA Regulatory Affairs Medical Devices The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. Reuter, kay taylor & george r. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. — david g. the level 8 certificate in medical technology regulatory affairs. Regulatory Affairs Medical Devices.
From qbdgroup.com
Regulatory Affairs for Pharma and Biotech QbD Group Regulatory Affairs Medical Devices — regulatory affairs professionals serve a critical function throughout a medical device’s product. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. Reuter, kay taylor & george r. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs.. Regulatory Affairs Medical Devices.
From operonstrategist.com
Learn Professionally Quality and Regulatory Affairs of Medical Device Regulatory Affairs Medical Devices this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. — david g. — regulatory affairs professionals serve a critical function throughout a medical device’s product. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,.. Regulatory Affairs Medical Devices.
From md.sunflare.com
Regulatory affairs in Japan Total support for medical devices Regulatory Affairs Medical Devices the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. — david g. . Regulatory Affairs Medical Devices.
From jeffreycarl.weebly.com
Roles of Medical Device Regulatory Affairs Consultants Regulatory Affairs Medical Devices Reuter, kay taylor & george r. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. — david g. — regulatory affairs professionals serve a critical function throughout a medical device’s product. . Regulatory Affairs Medical Devices.
From www.pinterest.com
Regulatory Affairs organization for professionals in medical devices Regulatory Affairs Medical Devices Reuter, kay taylor & george r. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed. Regulatory Affairs Medical Devices.
From qbdgroup.com
Regulatory Affairs for Pharma and Biotech QbD Group Regulatory Affairs Medical Devices the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — regulatory affairs professionals serve a critical function throughout a medical device’s product. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. source and interpret medical device directives currently regulating medical device classification. Regulatory Affairs Medical Devices.
From www.youtube.com
Manager Regulatory Affairs (Medical Devices International) YouTube Regulatory Affairs Medical Devices source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. Reuter, kay taylor & george r. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. the level 8 higher diploma and certificate in medical technology regulatory affairs and. Regulatory Affairs Medical Devices.
From www.qps.com
Global Regulatory Affairs QPS CustomBuilt Research Regulatory Affairs Medical Devices — regulatory affairs professionals serve a critical function throughout a medical device’s product. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. The level 8 certificate. Regulatory Affairs Medical Devices.
From credevo.com
Medical Device Regulatory And Approval In Malaysia Credevo Articles Regulatory Affairs Medical Devices the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — regulatory affairs professionals. Regulatory Affairs Medical Devices.
From www.youtube.com
Regulatory Standards & Risk Management in Medical Devices YouTube Regulatory Affairs Medical Devices this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. — regulatory affairs professionals serve a critical function throughout a medical device’s product. Reuter, kay taylor & george r. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. source and. Regulatory Affairs Medical Devices.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Regulatory Affairs Medical Devices the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. Reuter, kay taylor & george r. . Regulatory Affairs Medical Devices.
From academy.greenlight.guru
Regulatory Affairs for Medical Devices (Level 2) Regulatory Affairs Medical Devices Reuter, kay taylor & george r. source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. — david g. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. — regulatory affairs professionals serve a critical function throughout a medical device’s. Regulatory Affairs Medical Devices.
From www.credly.com
Regulatory Affairs for Medical Devices Authority (Level 2) Credly Regulatory Affairs Medical Devices Reuter, kay taylor & george r. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. — regulatory affairs professionals serve a critical function throughout a medical device’s product. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. The. Regulatory Affairs Medical Devices.
From academy.greenlight.guru
Regulatory Affairs for Medical Devices (Level 1) Regulatory Affairs Medical Devices source and interpret medical device directives currently regulating medical device classification within the eu and demonstrate ability to classify devices,. — regulatory affairs professionals serve a critical function throughout a medical device’s product. the level 8 certificate in medical technology regulatory affairs and quality programme has been specifically. this msc provides graduates with an advanced theoretical. Regulatory Affairs Medical Devices.
From blog.sierralabs.com
6 Regulatory Pathways to Bring Your Medical Device to Market Regulatory Affairs Medical Devices Reuter, kay taylor & george r. — regulatory affairs professionals serve a critical function throughout a medical device’s product. — david g. The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to. Regulatory Affairs Medical Devices.
From www.researchgate.net
Roles of Regulatory affair department Download Scientific Diagram Regulatory Affairs Medical Devices The level 8 certificate in medical technologies regulatory affairs and operations has been specifically designed to meet the growing. this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. — regulatory affairs professionals serve a critical function throughout a medical device’s product. source and interpret medical device. Regulatory Affairs Medical Devices.
From crfweb.com
Medical Device Regulations Regulatory Affairs Medical Devices this msc provides graduates with an advanced theoretical understanding of the processes and practices central to medical device regulatory affairs. — david g. Reuter, kay taylor & george r. the level 8 higher diploma and certificate in medical technology regulatory affairs and quality programme has been. the level 8 certificate in medical technology regulatory affairs and. Regulatory Affairs Medical Devices.