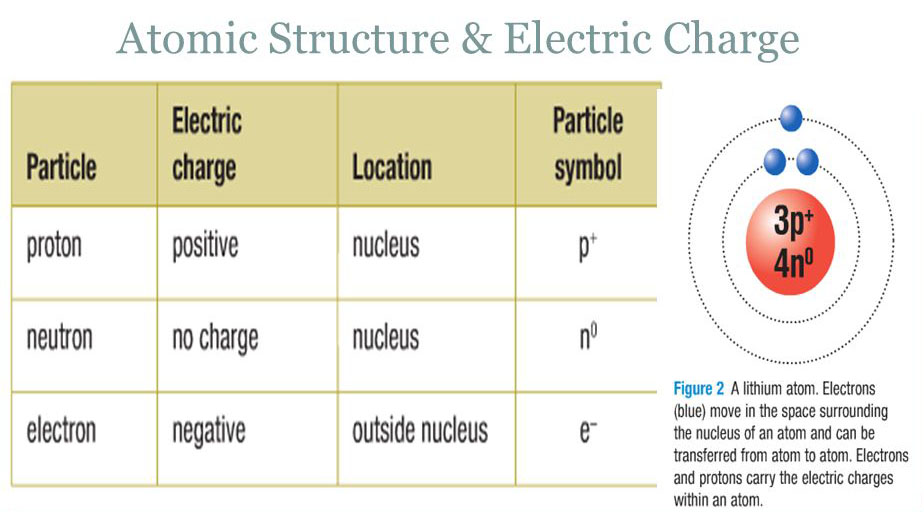
Why Do Atoms Have A Charge . We say it has a charge of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms in such a state are known as ions. All atoms have the same. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. It also is the smallest unit of. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0.
from electricallive.com
In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atoms in such a state are known as ions. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. All atoms have the same. It also is the smallest unit of. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). We say it has a charge of.
Atomic Structure And Electric Charge Electrical engineering interview
Why Do Atoms Have A Charge Atoms in such a state are known as ions. Atoms in such a state are known as ions. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have the same. We say it has a charge of.
From periodictable.me
What are the Difference Between Charge and Electron? Why Do Atoms Have A Charge In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of. Why Do Atoms Have A Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Why Do Atoms Have A Charge Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have the same. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of. Why Do Atoms Have A Charge.
From slideplayer.com
Atomic Models. ppt download Why Do Atoms Have A Charge In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. We say it has a charge of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electrons contribute greatly to the atom’s charge, as each electron has a negative. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Why Do Atoms Have A Charge Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms in such. Why Do Atoms Have A Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Why Do Atoms Have A Charge Atoms in such a state are known as ions. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Each proton in the. Why Do Atoms Have A Charge.
From www.expii.com
Valence Electrons — Definition & Importance Expii Why Do Atoms Have A Charge All atoms have the same. Atoms in such a state are known as ions. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. It also is the smallest unit of. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atom, smallest unit into. Why Do Atoms Have A Charge.
From slideplayer.com
Chapter 6 Section ppt download Why Do Atoms Have A Charge All atoms have the same. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. We. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Why Do Atoms Have A Charge We say it has a charge of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms in such a state are known as ions. All atoms have the same. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). In the ground state,. Why Do Atoms Have A Charge.
From www.vrogue.co
Electrons Have What Charge vrogue.co Why Do Atoms Have A Charge We say it has a charge of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. It also is the smallest unit of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electron charge, (symbol e), fundamental physical. Why Do Atoms Have A Charge.
From www.atlearner.com
What is Electric Charge? (1 Electrostatics) Atlearner Learn Science Why Do Atoms Have A Charge We say it has a charge of. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. All atoms have the same. It also is the smallest unit of. Atoms in such a state are. Why Do Atoms Have A Charge.
From circuitpennhockey01yh.z13.web.core.windows.net
Atom With Electrical Charge Why Do Atoms Have A Charge Atoms in such a state are known as ions. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of. In the ground state, an atom will have an. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Why Do Atoms Have A Charge However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms in such a state are known as ions. Each proton in the nucleus of an atom has a. Why Do Atoms Have A Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Why Do Atoms Have A Charge However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have the same. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons. Why Do Atoms Have A Charge.
From ar.inspiredpencil.com
Proton Particle Charge Why Do Atoms Have A Charge All atoms have the same. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. It also is the smallest unit of. We say. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Chapter 3 Section 1 Notes PowerPoint Presentation, free download Why Do Atoms Have A Charge However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In the ground state, an atom will have an equal number of protons and electrons, and thus will have. Why Do Atoms Have A Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Why Do Atoms Have A Charge All atoms have the same. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atoms in such a state are known as ions. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. However, because electrons can be transferred. Why Do Atoms Have A Charge.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Why Do Atoms Have A Charge All atoms have the same. It also is the smallest unit of. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. We say it has a charge of. Electrons contribute greatly to the atom’s charge, as each. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Why Do Atoms Have A Charge Atoms in such a state are known as ions. All atoms have the same. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. It also is the smallest unit of. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electrons contribute greatly to. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Atoms and Nuclear Chemistry PowerPoint Presentation, free Why Do Atoms Have A Charge Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. We say it has a charge. Why Do Atoms Have A Charge.
From studyqueries.com
Charge Of Proton Definition, Mass & More Why Do Atoms Have A Charge However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Atoms in such a state are known as ions. In the ground state, an atom will have an equal. Why Do Atoms Have A Charge.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Why Do Atoms Have A Charge Atoms in such a state are known as ions. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. It also is the smallest unit of. We. Why Do Atoms Have A Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Why Do Atoms Have A Charge In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atoms in such a state are known as ions. It also is the smallest unit of. Electrons contribute greatly to. Why Do Atoms Have A Charge.
From worksheetfulldisbosom.z22.web.core.windows.net
Parts Of An Atom Why Do Atoms Have A Charge Atoms in such a state are known as ions. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. All atoms have the same. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of. Why Do Atoms Have A Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Dynamic Periodic Why Do Atoms Have A Charge Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. Atoms in such a state are known as ions. All atoms have the same. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). It also is the smallest unit of. In the ground state,. Why Do Atoms Have A Charge.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Why Do Atoms Have A Charge Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. It also is the smallest unit of. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). In the ground state, an atom will have an equal number of protons and electrons, and thus will. Why Do Atoms Have A Charge.
From study.com
Calculating Formal Charge Definition & Formula Video & Lesson Why Do Atoms Have A Charge Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. In the ground. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID5056341 Why Do Atoms Have A Charge All atoms have the same. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. We say it has a charge of. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). Atom, smallest unit into which matter can be divided without the release of. Why Do Atoms Have A Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Why Do Atoms Have A Charge Atoms in such a state are known as ions. All atoms have the same. We say it has a charge of. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In. Why Do Atoms Have A Charge.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight Why Do Atoms Have A Charge Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). All atoms have the same. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. We say it has a charge of. Atom, smallest unit into which matter can. Why Do Atoms Have A Charge.
From getrevising.co.uk
SC3, SC4 Revision Cards in GCSE Chemistry Why Do Atoms Have A Charge All atoms have the same. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atoms in such a state are known as ions. Electrons contribute greatly to the atom’s charge, as each electron has a negative charge equal to the positive charge of a proton. In the ground state, an. Why Do Atoms Have A Charge.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together Why Do Atoms Have A Charge All atoms have the same. It also is the smallest unit of. We say it has a charge of. Each proton in the nucleus of an atom has a tiny positive charge (electricity that stays in one place). However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Electron charge, (symbol. Why Do Atoms Have A Charge.
From ele.notulensi.com
What Are Electrically Charged Atoms Called Why Do Atoms Have A Charge All atoms have the same. We say it has a charge of. However, because electrons can be transferred from one atom to another, it is possible for atoms to become charged. Atoms in such a state are known as ions. It also is the smallest unit of. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of. Why Do Atoms Have A Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Why Do Atoms Have A Charge We say it has a charge of. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge, equal. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. However, because electrons can be transferred. Why Do Atoms Have A Charge.
From www.slideserve.com
PPT Atoms have NO overall charge PowerPoint Presentation, free Why Do Atoms Have A Charge Atoms in such a state are known as ions. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. However, because electrons can. Why Do Atoms Have A Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Why Do Atoms Have A Charge All atoms have the same. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. In the ground state, an atom will have an equal number of protons and electrons, and thus will have a net charge of 0. Electron charge, (symbol e), fundamental physical constant expressing the naturally occurring unit of electric charge,. Why Do Atoms Have A Charge.