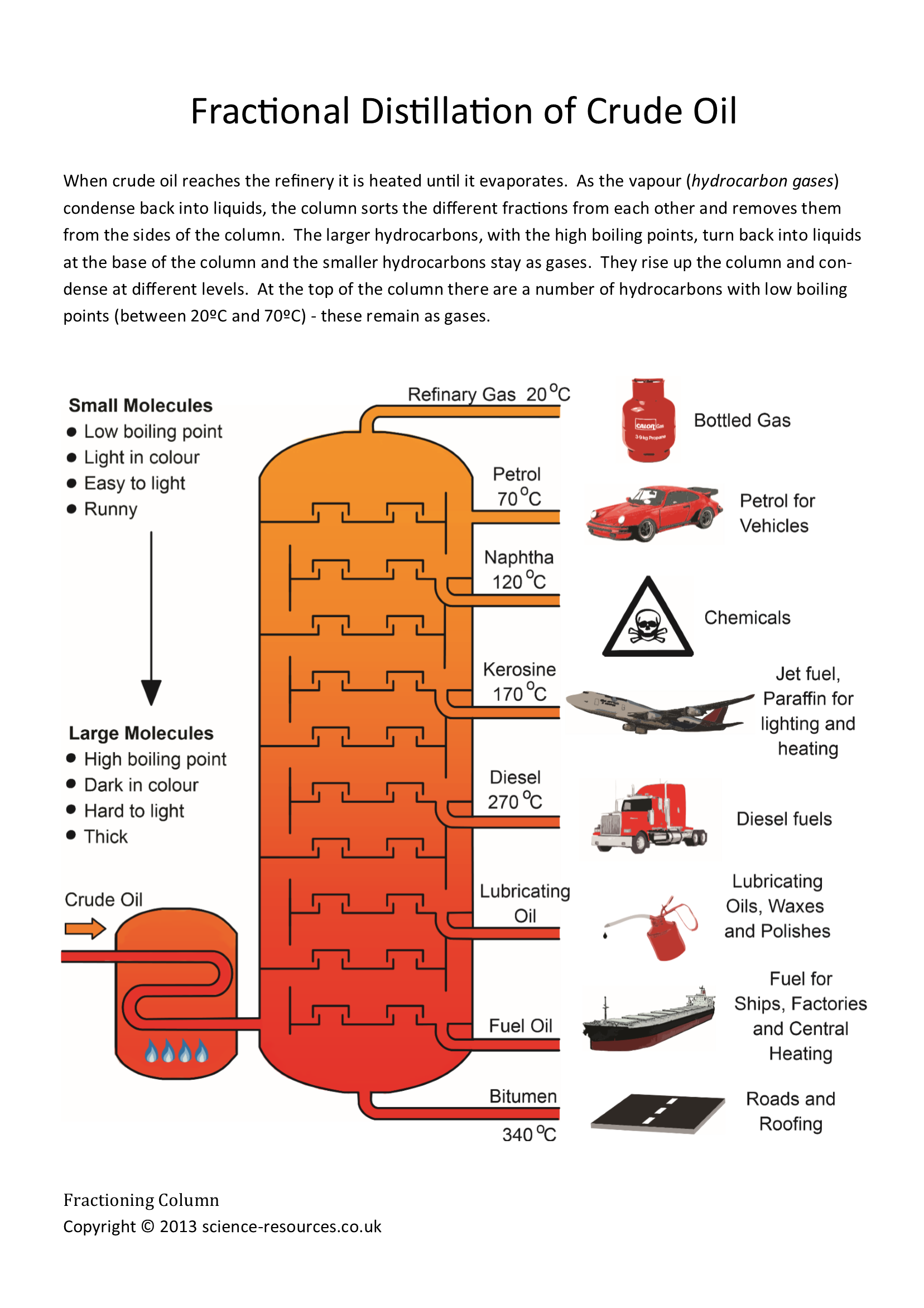
Oil Chemistry Uses . Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. There are many types, such as essential oil;. This section is a simplified introduction to oil and petroleum chemistry and the properties. Oil, any greasy substance that is liquid at room temperature and insoluble in water. Petroleum contains four classes of compound: Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. * lubricating oil comes from heavier molecules with 20 to 50 carbons. * fuel oil has roughly 20 to 70 carbons per molecule and finds. The basic composition of oil is.
from www.pinterest.com
Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. This section is a simplified introduction to oil and petroleum chemistry and the properties. Petroleum contains four classes of compound: Oil, any greasy substance that is liquid at room temperature and insoluble in water. * fuel oil has roughly 20 to 70 carbons per molecule and finds. There are many types, such as essential oil;.
Fractional Distillation of Crude Oil Poster Science Chemistry
Oil Chemistry Uses Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Oil, any greasy substance that is liquid at room temperature and insoluble in water. Petroleum contains four classes of compound: Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. This section is a simplified introduction to oil and petroleum chemistry and the properties. * fuel oil has roughly 20 to 70 carbons per molecule and finds. The basic composition of oil is. * lubricating oil comes from heavier molecules with 20 to 50 carbons. There are many types, such as essential oil;.
From mybios.me
Chemical Position Of Vegetable Oil My Bios Oil Chemistry Uses Petroleum contains four classes of compound: Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Oil, any greasy. Oil Chemistry Uses.
From www.tes.com
GCSE Core Chemistry Revision Lessons Bundle Teaching Resources Oil Chemistry Uses Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. The basic composition of oil is. * fuel oil has roughly 20 to 70 carbons per molecule and finds. * lubricating. Oil Chemistry Uses.
From www.youtube.com
Crude Oil Fractions & Their Uses Organic Chemistry Chemistry Oil Chemistry Uses Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. This section is a simplified introduction to oil and petroleum chemistry and the properties. Oil, any greasy substance. Oil Chemistry Uses.
From ar.inspiredpencil.com
Chemistry Of Oil Oil Chemistry Uses Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. This section is a simplified introduction to oil and petroleum chemistry and the properties. Petroleum contains four classes of compound: * fuel oil has roughly 20 to 70 carbons per molecule and finds. There are many types, such as essential oil;. * lubricating oil comes from heavier molecules with 20. Oil Chemistry Uses.
From sherpa-online.com
GCSE Chemistry Crude Oil and Hydrocarbons, what is Crude Oil and how Oil Chemistry Uses The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Petroleum contains four classes of compound: Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. This section. Oil Chemistry Uses.
From www.researchgate.net
Composition of crude oil Download Scientific Diagram Oil Chemistry Uses * lubricating oil comes from heavier molecules with 20 to 50 carbons. The basic composition of oil is. There are many types, such as essential oil;. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more.. Oil Chemistry Uses.
From www.pinterest.com
Fractional Distillation of Crude Oil Poster Science Chemistry Oil Chemistry Uses Petroleum contains four classes of compound: Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. The basic composition of oil is. Oil, any greasy substance that is. Oil Chemistry Uses.
From www.ic2r.com
Oil Chemistry IC2R Ltd Oil Chemistry Uses * lubricating oil comes from heavier molecules with 20 to 50 carbons. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. This section is a simplified introduction to oil and petroleum chemistry and the properties. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used. Oil Chemistry Uses.
From en.ppt-online.org
Physical and chemical properties of oil online presentation Oil Chemistry Uses Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical. Oil Chemistry Uses.
From www.edibleoilrefinerymachine.com
Edible Oil Physical Refining vs Edible Oil Chemical Refining_Tech Oil Chemistry Uses Oil, any greasy substance that is liquid at room temperature and insoluble in water. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. This section is a simplified introduction to oil and petroleum chemistry and the properties. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. *. Oil Chemistry Uses.
From www.pinterest.com
18 best Essential Oil Chemistry images on Pinterest Essential oil Oil Chemistry Uses Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. There are many types, such as essential oil;. Over time, heat and pressure allowed chemical reactions where the relatively simple. Oil Chemistry Uses.
From www.pinterest.com
Essential Oil Chemistry Essential oils, Chemistry, Aromatherapy blends Oil Chemistry Uses Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. This section is a simplified introduction to oil and petroleum chemistry and the properties. * fuel oil has roughly 20 to 70 carbons per molecule and finds. Oil, any greasy substance that is liquid at room. Oil Chemistry Uses.
From www.youtube.com
Petroleum and its refining Chemistry YouTube Oil Chemistry Uses Oil, any greasy substance that is liquid at room temperature and insoluble in water. * fuel oil has roughly 20 to 70 carbons per molecule and finds. There are many types, such as essential oil;. This section is a simplified introduction to oil and petroleum chemistry and the properties. The basic composition of oil is. Petroleum contains four classes of. Oil Chemistry Uses.
From www.shalom-education.com
Fractional Distillation and Crude Oil GCSE Chemistry Revision Oil Chemistry Uses There are many types, such as essential oil;. The basic composition of oil is. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. * lubricating oil comes from heavier molecules. Oil Chemistry Uses.
From chem.libretexts.org
13.2 Fats and Oils Chemistry LibreTexts Oil Chemistry Uses Oil, any greasy substance that is liquid at room temperature and insoluble in water. The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. There are many types, such as essential oil;. * lubricating oil comes from heavier molecules with. Oil Chemistry Uses.
From www.slideserve.com
PPT Basic Chemistry of Biodiesel Production p resented at CCURI Oil Chemistry Uses Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. The basic composition of oil is. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. * fuel oil has roughly 20 to 70 carbons per molecule and finds. Unlike coal, which is primarily used as. Oil Chemistry Uses.
From ar.inspiredpencil.com
Chemistry Of Oil Oil Chemistry Uses The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Oil, any greasy substance that is liquid at room temperature and insoluble in water. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen,. Oil Chemistry Uses.
From www.researchgate.net
(PDF) Introduction to Oil Chemistry and Properties Related to Oil Spills Oil Chemistry Uses * lubricating oil comes from heavier molecules with 20 to 50 carbons. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. This section is a simplified introduction to oil and petroleum chemistry and the properties. The basic composition of oil is. Petroleum contains four classes of compound: Oil,. Oil Chemistry Uses.
From www.slideserve.com
PPT Natural Fats and Oils PowerPoint Presentation, free download ID Oil Chemistry Uses The basic composition of oil is. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. This section is a simplified introduction to oil and petroleum chemistry and the properties. Oil, any greasy substance that is liquid at room temperature and insoluble in water. * fuel oil has roughly 20 to 70 carbons per molecule and finds. * lubricating oil. Oil Chemistry Uses.
From crudeoildatsuhata.blogspot.com
Crude Oil The Fractional Distillation Of Crude Oil Oil Chemistry Uses Petroleum contains four classes of compound: There are many types, such as essential oil;. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen. Oil Chemistry Uses.
From blog.enerpac.com
How Crude Oil is Separated Into Fractions Enerpac Blog Oil Chemistry Uses * fuel oil has roughly 20 to 70 carbons per molecule and finds. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen,. Oil Chemistry Uses.
From www.reddit.com
The chemistry of petrol and diesel r/chemistry Oil Chemistry Uses Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Petroleum contains four classes of compound: Oil, any greasy substance that is liquid at room temperature and insoluble in water. The basic composition of oil is. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a. Oil Chemistry Uses.
From www.youtube.com
Essential Oil Chemistry Introduction to Essential Oils Chemical Oil Chemistry Uses Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. There are many types, such as essential oil;. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. The basic composition of oil is. Over time, heat. Oil Chemistry Uses.
From mavink.com
Chemical Structure Of Oil Oil Chemistry Uses There are many types, such as essential oil;. Petroleum contains four classes of compound: * lubricating oil comes from heavier molecules with 20 to 50 carbons. * fuel oil has roughly 20 to 70 carbons per molecule and finds. The basic composition of oil is. This section is a simplified introduction to oil and petroleum chemistry and the properties. Unlike. Oil Chemistry Uses.
From igcseandialchemistry.com
Uses of crude oil and its separtation Oil Chemistry Uses Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. This section is a simplified introduction to oil and petroleum chemistry and the properties. There are many types, such as essential oil;. * fuel oil has roughly 20 to 70 carbons per molecule and finds. The basic composition of oil is. Petroleum. Oil Chemistry Uses.
From www.pinterest.com
How fractional distillation of crude oil works. Fractional Oil Chemistry Uses Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. This section is a simplified introduction to oil and petroleum chemistry and the properties. Petroleum contains four classes of compound: Oil, any greasy substance that is liquid at room temperature and insoluble in water. The basic composition of oil. Oil Chemistry Uses.
From www.pinterest.com
The Chemistry of Essential Oil Reveals the Properties of the Oil Oil Chemistry Uses Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one or more. There are many types, such as essential oil;. Petroleum contains four classes of compound: The basic composition of oil is. * lubricating oil comes from heavier molecules with 20 to 50 carbons.. Oil Chemistry Uses.
From passmyexams.co.uk
Crude Oil / Petroleum Easy exam revision notes for GSCE Chemistry Oil Chemistry Uses Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. There are many types, such as essential oil;. * fuel oil has roughly 20 to 70 carbons per molecule and finds. Oil, any greasy substance that is liquid at room temperature and insoluble in water. This section is a. Oil Chemistry Uses.
From chemistnotes.com
Essential oil Definition, Preparation, and 5 Reliable Uses Chemistry Oil Chemistry Uses Petroleum contains four classes of compound: Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. The basic composition of oil is. This section is a simplified introduction to oil and petroleum chemistry and the properties. Oil, any greasy substance that is liquid at room temperature. Oil Chemistry Uses.
From www.pinterest.com.mx
Pin on H11 Oil Chemistry Uses Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. * lubricating oil comes from heavier molecules with 20 to 50 carbons. Petroleum contains four classes of compound: Alkanes, cycloalkanes, aromatics,. Oil Chemistry Uses.
From www.researchgate.net
Physical and chemical properties of oils. Download Table Oil Chemistry Uses Unlike coal, which is primarily used as a fuel for electricity generation, oil is primarily used as a fuel for transportation. This section is a simplified introduction to oil and petroleum chemistry and the properties. The basic composition of oil is. Petroleum contains four classes of compound: Over time, heat and pressure allowed chemical reactions where the relatively simple combinations. Oil Chemistry Uses.
From www.slideserve.com
PPT Sources of Fats and Oils PowerPoint Presentation, free download Oil Chemistry Uses The basic composition of oil is. This section is a simplified introduction to oil and petroleum chemistry and the properties. * fuel oil has roughly 20 to 70 carbons per molecule and finds. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Since oil is made mostly out of carbon and. Oil Chemistry Uses.
From aromahut.com
Essential Oil Chemistry Cheat Sheets Oil Chemistry Uses Oil, any greasy substance that is liquid at room temperature and insoluble in water. This section is a simplified introduction to oil and petroleum chemistry and the properties. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Since oil is made mostly out of carbon and hydrogen atoms, it is known. Oil Chemistry Uses.
From www.saubhaya.com
Chemical Makeup Of Oil Saubhaya Makeup Oil Chemistry Uses Petroleum contains four classes of compound: This section is a simplified introduction to oil and petroleum chemistry and the properties. Over time, heat and pressure allowed chemical reactions where the relatively simple combinations of carbon, hydrogen, and oxygen. Oil, any greasy substance that is liquid at room temperature and insoluble in water. Alkanes, cycloalkanes, aromatics, and heteroatomic compounds with one. Oil Chemistry Uses.
From owlcation.com
Making Crude Oil Useful Fractional Distillation and Cracking Owlcation Oil Chemistry Uses The basic composition of oil is. * lubricating oil comes from heavier molecules with 20 to 50 carbons. * fuel oil has roughly 20 to 70 carbons per molecule and finds. Since oil is made mostly out of carbon and hydrogen atoms, it is known as a hydrocarbon (although from a chemical standpoint, it's often. Alkanes, cycloalkanes, aromatics, and heteroatomic. Oil Chemistry Uses.