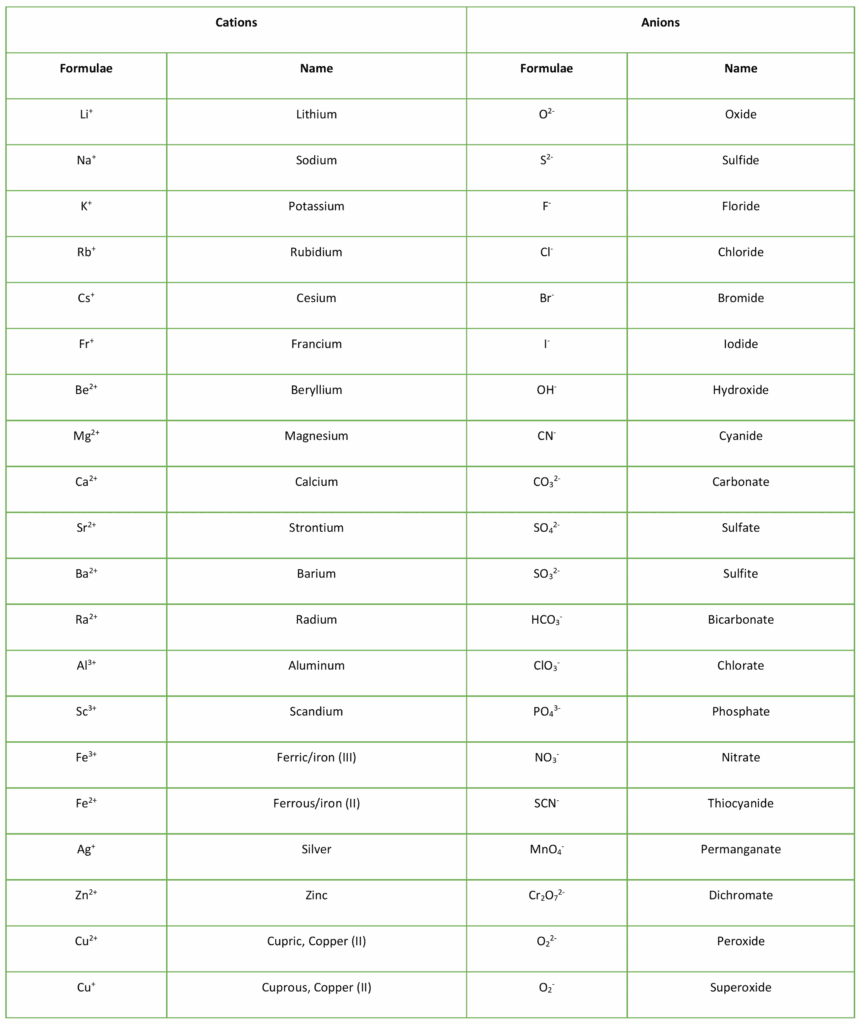
Br Cation Or Anion . It is a conjugate base of a hydrogen bromide. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Cations and anions are the two types of ions. Because most metals form cations and most nonmetals form anions, formulas typically. (cations and anions) cations 1+ ammonium nh 4 + cesium. Bromide is a halide anion and a monoatomic bromine. Electrons can move from one atom to another; Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. In nature, bromine is most. First, the cation is written before the anion. When they do, species with overall electric charges are formed. As with simple ionic compounds, these compounds must also.
from psiberg.com
Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Cations and anions are the two types of ions. It is a conjugate base of a hydrogen bromide. Electrons can move from one atom to another; (cations and anions) cations 1+ ammonium nh 4 + cesium. Because most metals form cations and most nonmetals form anions, formulas typically. First, the cation is written before the anion. In nature, bromine is most. As with simple ionic compounds, these compounds must also.
Cation vs. Anion Differences and Examples PSIBERG
Br Cation Or Anion When they do, species with overall electric charges are formed. In nature, bromine is most. Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. As with simple ionic compounds, these compounds must also. (cations and anions) cations 1+ ammonium nh 4 + cesium. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. It is a conjugate base of a hydrogen bromide. Cations and anions are the two types of ions. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. First, the cation is written before the anion. Bromide is a halide anion and a monoatomic bromine. Electrons can move from one atom to another;
From karsyntinoconnell.blogspot.com
Cations and Anions List KarsyntinOconnell Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. As with simple ionic compounds, these compounds must also. First, the cation is written before the anion. In nature, bromine is most. Bromide is a halide anion and a monoatomic bromine. Electrons can move from one atom to another; When they do, species with. Br Cation Or Anion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Br Cation Or Anion In nature, bromine is most. Electrons can move from one atom to another; Because most metals form cations and most nonmetals form anions, formulas typically. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Bromide is a halide anion and a monoatomic bromine. As with simple ionic compounds, these compounds must also. (cations and anions) cations. Br Cation Or Anion.
From slideplayer.com
Elements and Ions. ppt download Br Cation Or Anion Because most metals form cations and most nonmetals form anions, formulas typically. Electrons can move from one atom to another; Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. (cations and anions) cations 1+ ammonium nh 4 + cesium. As. Br Cation Or Anion.
From www.geeksforgeeks.org
Cations and Anions Difference between Cations and Anions Br Cation Or Anion Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Cations and anions are the two types of ions. As with simple ionic compounds, these compounds must also. It is a conjugate. Br Cation Or Anion.
From www.youtube.com
Anions & Cations Trick/ Mnemonic to remember Difference Examples Br Cation Or Anion (cations and anions) cations 1+ ammonium nh 4 + cesium. As with simple ionic compounds, these compounds must also. When they do, species with overall electric charges are formed. Because most metals form cations and most nonmetals form anions, formulas typically. Cations and anions are the two types of ions. Many ionic compounds contain polyatomic ions as the cation, the. Br Cation Or Anion.
From psiberg.com
Cation vs. Anion Differences and Examples PSIBERG Br Cation Or Anion Electrons can move from one atom to another; Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. As with simple ionic compounds, these compounds must also. It is a conjugate base of a hydrogen bromide. In nature, bromine is most. Bromide is a halide anion and a monoatomic bromine. When they do, species. Br Cation Or Anion.
From www.bartleby.com
Answered Table 12.3 Ionic Radii for Several… bartleby Br Cation Or Anion (cations and anions) cations 1+ ammonium nh 4 + cesium. Bromide is a halide anion and a monoatomic bromine. As with simple ionic compounds, these compounds must also. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Cations and anions are the two types of ions. When they do, species with overall electric charges are formed.. Br Cation Or Anion.
From adrienj.tinosmarble.com
Cations vs Anions Difference Between Cations and Anions with Examples Br Cation Or Anion In nature, bromine is most. When they do, species with overall electric charges are formed. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Because most metals form cations and most nonmetals form anions, formulas typically. Cations and anions are. Br Cation Or Anion.
From www.numerade.com
SOLVED the table below by writing the symbols for the cation Br Cation Or Anion First, the cation is written before the anion. It is a conjugate base of a hydrogen bromide. Because most metals form cations and most nonmetals form anions, formulas typically. Bromide is a halide anion and a monoatomic bromine. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Electrons can move from one atom to another; When. Br Cation Or Anion.
From www.lookfordiagnosis.com
Cations Br Cation Or Anion As with simple ionic compounds, these compounds must also. (cations and anions) cations 1+ ammonium nh 4 + cesium. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Cations and anions are the two types of ions. Electrons can move from one atom to another; In nature, bromine is most. First, the cation is written before. Br Cation Or Anion.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 Br Cation Or Anion Electrons can move from one atom to another; As with simple ionic compounds, these compounds must also. Cations and anions are the two types of ions. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. It is a conjugate base of a hydrogen bromide. Bromide is a halide anion and a monoatomic bromine. In nature, bromine. Br Cation Or Anion.
From waterfilterguru.com
Cation Exchange vs Anion Exchange What's the Difference? Br Cation Or Anion It is a conjugate base of a hydrogen bromide. As with simple ionic compounds, these compounds must also. Cations and anions are the two types of ions. In nature, bromine is most. Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. Many ionic compounds contain polyatomic ions. Br Cation Or Anion.
From slideplayer.com
Nomenclature. ppt download Br Cation Or Anion First, the cation is written before the anion. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. In nature, bromine is most. When they do, species with overall electric charges are formed. As with simple ionic compounds, these compounds must also. Many ionic compounds contain polyatomic ions as the cation, the anion, or. Br Cation Or Anion.
From www.geeksforgeeks.org
Cations and Anions Difference between Cations and Anions Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. (cations and anions) cations 1+ ammonium nh 4 + cesium. First, the cation is written before the anion. It is a conjugate base of a hydrogen bromide. Electrons can move from. Br Cation Or Anion.
From www.getbodysmart.com
Cations and Anions examples and diagrams GetBodySmart Br Cation Or Anion In nature, bromine is most. (cations and anions) cations 1+ ammonium nh 4 + cesium. Cations and anions are the two types of ions. Electrons can move from one atom to another; Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. First, the cation is written before. Br Cation Or Anion.
From www.coursehero.com
[Solved] Identify each as a cation, an anion, or neither. NH 3 Br − H − Br Cation Or Anion Bromide is a halide anion and a monoatomic bromine. Because most metals form cations and most nonmetals form anions, formulas typically. First, the cation is written before the anion. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. As with. Br Cation Or Anion.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 Br Cation Or Anion When they do, species with overall electric charges are formed. First, the cation is written before the anion. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Because most metals form cations and most nonmetals form anions, formulas typically. In nature, bromine is most. Cations and anions are the two types of ions.. Br Cation Or Anion.
From es.scribd.com
Cation+Anion+Nombre+[Br] Átomos Conjuntos de elementos químicos Br Cation Or Anion As with simple ionic compounds, these compounds must also. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Bromide is a halide anion and a monoatomic bromine. First, the cation is written before the anion. (cations and anions) cations 1+ ammonium nh 4 + cesium. In nature, bromine is most. Electrons can move from one atom. Br Cation Or Anion.
From www.youtube.com
what is an Ion? Cation and Anion Chemistry YouTube Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Bromide is a halide anion and a monoatomic bromine. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. First, the cation is written before the anion. When they do, species with overall electric charges are formed. It is a conjugate. Br Cation Or Anion.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Br Cation Or Anion Bromide is a halide anion and a monoatomic bromine. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. First, the cation is written before the anion. When they do, species with overall electric charges are formed. Electrons can move from. Br Cation Or Anion.
From www.thoughtco.com
The Difference Between a Cation and an Anion Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Electrons can move from one atom to another; Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. As. Br Cation Or Anion.
From pediaa.com
Difference Between Cation and Anion Br Cation Or Anion (cations and anions) cations 1+ ammonium nh 4 + cesium. When they do, species with overall electric charges are formed. Bromide is a halide anion and a monoatomic bromine. It is a conjugate base of a hydrogen bromide. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions have an imbalance of electrical charge, meaning they. Br Cation Or Anion.
From www.slideshare.net
Ionic Bonding Notes Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Bromide is a halide anion and a monoatomic bromine. In nature, bromine is most. Cations and anions are the two types of ions. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. (cations and anions) cations 1+ ammonium nh 4. Br Cation Or Anion.
From basichemistry.blogspot.com
Basic Chemistry Binary Compounds Type I Br Cation Or Anion Bromide is a halide anion and a monoatomic bromine. First, the cation is written before the anion. Because most metals form cations and most nonmetals form anions, formulas typically. As with simple ionic compounds, these compounds must also. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. It is a conjugate base of. Br Cation Or Anion.
From www.aquaportail.com
Anion définition et explications Br Cation Or Anion When they do, species with overall electric charges are formed. Because most metals form cations and most nonmetals form anions, formulas typically. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Electrons can move from one atom to another; Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. It. Br Cation Or Anion.
From www.youtube.com
How to Draw the Lewis Dot Structure for Br (Bromide ion) YouTube Br Cation Or Anion First, the cation is written before the anion. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. It is a conjugate base of a hydrogen bromide. In nature, bromine is most. As with simple ionic compounds, these compounds must also. Bromide is a halide anion and a monoatomic bromine. (cations and anions) cations 1+ ammonium nh. Br Cation Or Anion.
From www.youtube.com
Cation vs Anion What's the difference?! YouTube Br Cation Or Anion When they do, species with overall electric charges are formed. First, the cation is written before the anion. Cations and anions are the two types of ions. Bromide is a halide anion and a monoatomic bromine. Because most metals form cations and most nonmetals form anions, formulas typically. As with simple ionic compounds, these compounds must also. Electrons can move. Br Cation Or Anion.
From animalia-life.club
Cation And Anion Br Cation Or Anion Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. (cations and anions) cations 1+ ammonium nh 4 + cesium. First, the cation is written before the anion. As with simple ionic compounds, these compounds must also. Cations and anions are the two types of ions. Because most metals form cations and most nonmetals. Br Cation Or Anion.
From www.researchgate.net
Arrangement of four C5N2H14 2+ cations around the Br − anion. Bromine Br Cation Or Anion Because most metals form cations and most nonmetals form anions, formulas typically. It is a conjugate base of a hydrogen bromide. First, the cation is written before the anion. Bromide is a halide anion and a monoatomic bromine. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. Ions have an imbalance of electrical charge, meaning they. Br Cation Or Anion.
From www.difference101.com
Cation vs. Anion 7 Key Differences, Pros & Cons, Examples Difference 101 Br Cation Or Anion It is a conjugate base of a hydrogen bromide. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. When they do, species with overall electric charges are formed. Because most metals form cations and most nonmetals form anions, formulas typically. Electrons can move from one atom to another; Cations and anions are the. Br Cation Or Anion.
From www.slideshare.net
Ionic bonding binary Br Cation Or Anion Because most metals form cations and most nonmetals form anions, formulas typically. When they do, species with overall electric charges are formed. First, the cation is written before the anion. In nature, bromine is most. Electrons can move from one atom to another; Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Cations. Br Cation Or Anion.
From www.bartleby.com
Answered Cation Formula Anion Formula Compound… bartleby Br Cation Or Anion Bromide is a halide anion and a monoatomic bromine. In nature, bromine is most. Electrons can move from one atom to another; Because most metals form cations and most nonmetals form anions, formulas typically. As with simple ionic compounds, these compounds must also. Many ionic compounds contain polyatomic ions as the cation, the anion, or both. It is a conjugate. Br Cation Or Anion.
From www.youtube.com
Cations and Anions Explained YouTube Br Cation Or Anion As with simple ionic compounds, these compounds must also. Electrons can move from one atom to another; Cations and anions are the two types of ions. Ions have an imbalance of electrical charge, meaning they contain different numbers of protons and electrons. Because most metals form cations and most nonmetals form anions, formulas typically. Bromide is a halide anion and. Br Cation Or Anion.
From sciencenotes.org
Common Anions List and Formulas Br Cation Or Anion Cations and anions are the two types of ions. Electrons can move from one atom to another; As with simple ionic compounds, these compounds must also. In nature, bromine is most. (cations and anions) cations 1+ ammonium nh 4 + cesium. Bromide is a halide anion and a monoatomic bromine. Ions have an imbalance of electrical charge, meaning they contain. Br Cation Or Anion.
From animalia-life.club
Cation And Anion Br Cation Or Anion Bromide is a halide anion and a monoatomic bromine. As with simple ionic compounds, these compounds must also. Electrons can move from one atom to another; Many ionic compounds contain polyatomic ions as the cation, the anion, or both. It is a conjugate base of a hydrogen bromide. In nature, bromine is most. (cations and anions) cations 1+ ammonium nh. Br Cation Or Anion.