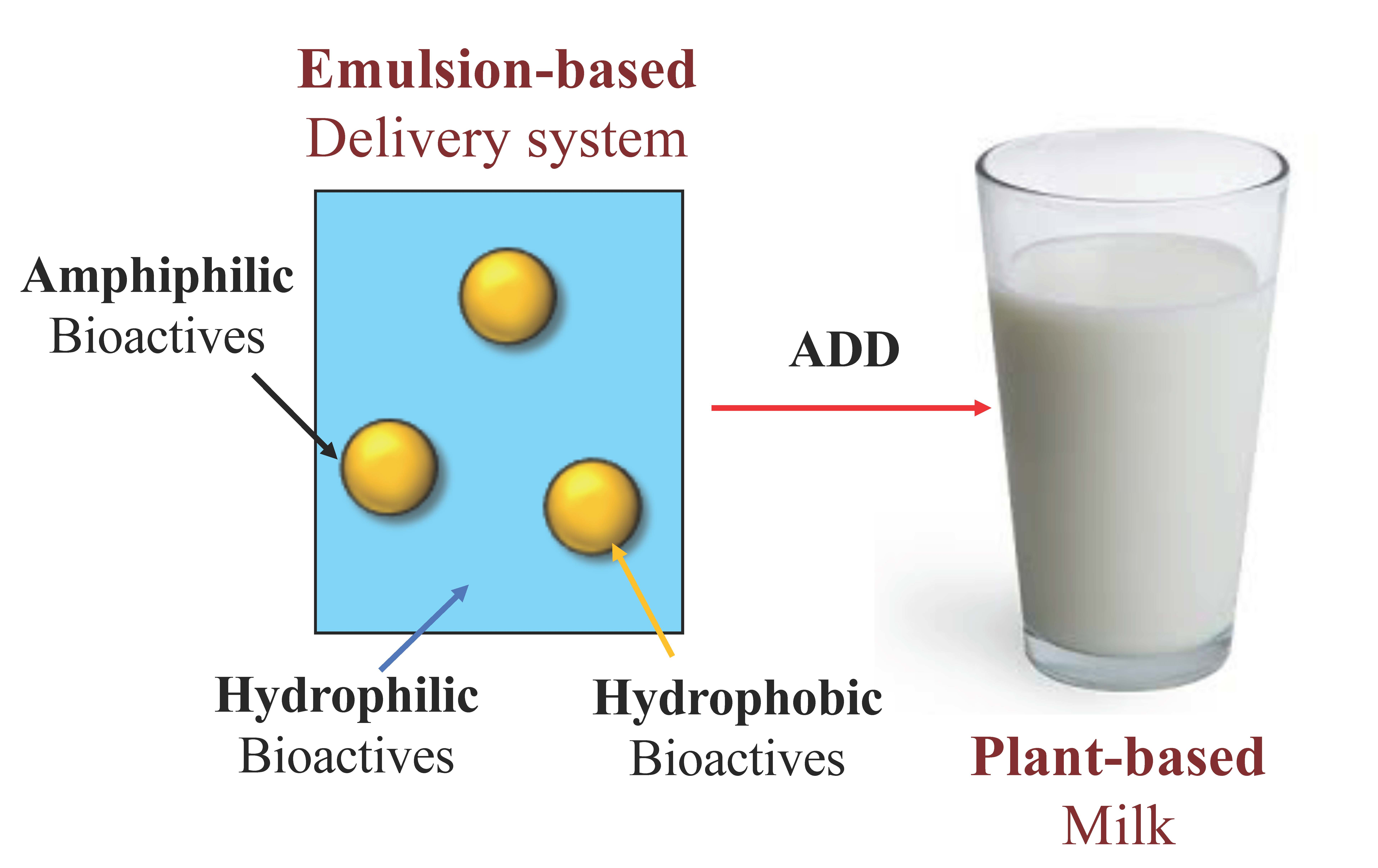
Is Chocolate Milk A Solution Colloid Or Suspension . A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A solution may be colored, but it is transparent, the molecules or ions are. (c) a suspension, such as mud, is a. If you let a suspension sit for long enough, however, the particles will. Examples would be fog and milk (colloids); Solutions exhibit completely different behavior from suspensions. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. And freshly stirred paint and hot chocolate (suspensions). Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a solution, and explore solution, suspension, and colloid examples. Compare colloids, suspensions, solutions, and find their differences.
from www.animalia-life.club
Examples would be fog and milk (colloids); Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Solutions exhibit completely different behavior from suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Understand particles in a solution, and explore solution, suspension, and colloid examples. And freshly stirred paint and hot chocolate (suspensions). (c) a suspension, such as mud, is a. Compare colloids, suspensions, solutions, and find their differences. If you let a suspension sit for long enough, however, the particles will. A solution may be colored, but it is transparent, the molecules or ions are.
Colloid Milk
Is Chocolate Milk A Solution Colloid Or Suspension Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (c) a suspension, such as mud, is a. Understand particles in a solution, and explore solution, suspension, and colloid examples. Examples would be fog and milk (colloids); If you let a suspension sit for long enough, however, the particles will. And freshly stirred paint and hot chocolate (suspensions). A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. Compare colloids, suspensions, solutions, and find their differences. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension.
From slideplayer.com
Elements, Compounds, Solutions, Colloids, & Suspensions ppt download Is Chocolate Milk A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Examples would be fog and milk (colloids); And freshly stirred paint and hot chocolate (suspensions). (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. A solution may. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.pinterest.ph
Solutions Colloids and Suspensions Worksheet Fresh solutions Colloids Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. A solution may be colored, but it is transparent, the molecules or ions are. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (c) a suspension, such as mud, is a. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.slideserve.com
PPT COLLOID PowerPoint Presentation ID1550604 Is Chocolate Milk A Solution Colloid Or Suspension Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (c) a suspension, such as mud, is a. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. And. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Is Chocolate Milk A Solution Colloid Or Suspension (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. And freshly stirred paint and hot chocolate (suspensions). Compare colloids, suspensions, solutions, and find their differences. If you let a suspension sit for long enough, however, the particles will. A solution may be colored, but it is transparent, the molecules or. Is Chocolate Milk A Solution Colloid Or Suspension.
From ar.inspiredpencil.com
Colloid Suspension Solution Is Chocolate Milk A Solution Colloid Or Suspension (c) a suspension, such as mud, is a. A solution may be colored, but it is transparent, the molecules or ions are. If you let a suspension sit for long enough, however, the particles will. And freshly stirred paint and hot chocolate (suspensions). Understand particles in a solution, and explore solution, suspension, and colloid examples. Solutions exhibit completely different behavior. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Examples would be fog and milk (colloids); A solution may be colored, but it is transparent, the molecules or ions are. And freshly stirred paint and hot chocolate (suspensions). (b) in a colloid, such as milk,. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Chapter 16 Homogeneous Mixtures Solutions & Colloids Stuff Dissolved Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. (c) a suspension, such as mud, is a. Solutions exhibit completely different behavior from suspensions. If you let a suspension sit for long enough, however, the particles will. And freshly stirred paint and hot chocolate (suspensions). Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger.. Is Chocolate Milk A Solution Colloid Or Suspension.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. A solution may be colored, but it is transparent, the molecules or ions are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is. Is Chocolate Milk A Solution Colloid Or Suspension.
From foodsalternative.com
Is Milk a Colloid, Suspension Compound, or a Mixture? Why Is Chocolate Milk A Solution Colloid Or Suspension Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a solution, and explore solution, suspension, and colloid examples. A solution may be colored, but it is transparent, the molecules or ions are. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
HETEROGENEOUS AQUEOUS SYSTEMS Chapter OBJECTIVE I will be able to Is Chocolate Milk A Solution Colloid Or Suspension (c) a suspension, such as mud, is a. If you let a suspension sit for long enough, however, the particles will. Compare colloids, suspensions, solutions, and find their differences. And freshly stirred paint and hot chocolate (suspensions). A solution may be colored, but it is transparent, the molecules or ions are. A colloid is a heterogeneous mixture in which the. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.animalia-life.club
Colloid Milk Is Chocolate Milk A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A solution may be colored, but it is transparent, the molecules or ions are. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. And freshly stirred paint. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.animalia-life.club
Colloid Milk Is Chocolate Milk A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are. Compare colloids, suspensions, solutions, and find their differences. Solutions exhibit completely different behavior from suspensions. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. If you let a suspension sit for long enough, however, the particles. Is Chocolate Milk A Solution Colloid Or Suspension.
From foodsalternative.com
Is Milk a Colloid, Suspension Compound, or a Mixture? Why Is Chocolate Milk A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Understand particles in a solution, and explore solution, suspension, and colloid examples. (c) a suspension, such as mud, is a. And freshly stirred. Is Chocolate Milk A Solution Colloid Or Suspension.
From en.wikipedia.org
Colloid Wikipedia Is Chocolate Milk A Solution Colloid Or Suspension And freshly stirred paint and hot chocolate (suspensions). A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (b) in a colloid, such as milk, the particles are much larger but. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Is Chocolate Milk A Solution Colloid Or Suspension If you let a suspension sit for long enough, however, the particles will. And freshly stirred paint and hot chocolate (suspensions). (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Solutions exhibit completely different behavior from suspensions. Compare colloids, suspensions, solutions, and find their differences. A solution may be colored,. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Is Chocolate Milk A Solution Colloid Or Suspension And freshly stirred paint and hot chocolate (suspensions). Examples would be fog and milk (colloids); (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. If you let a suspension sit for long enough, however, the particles will. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate. Is Chocolate Milk A Solution Colloid Or Suspension.
From foodsalternative.com
Is Milk a Colloid, Suspension Compound, or a Mixture? Why Is Chocolate Milk A Solution Colloid Or Suspension Understand particles in a solution, and explore solution, suspension, and colloid examples. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. And freshly stirred paint and hot chocolate (suspensions). Examples would be fog and milk (colloids); Solutions exhibit completely different behavior from suspensions. A solution may be colored, but it. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Is Chocolate Milk A Solution Colloid Or Suspension (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. And freshly stirred paint and hot chocolate (suspensions). If you let a suspension sit for long enough, however, the particles will. Solutions exhibit completely different behavior from suspensions. Examples would be fog and milk (colloids); (c) a suspension, such as mud,. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Is Chocolate Milk A Solution Colloid Or Suspension (c) a suspension, such as mud, is a. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a solution, and explore solution, suspension, and colloid examples. (b). Is Chocolate Milk A Solution Colloid Or Suspension.
From www.showme.com
Solutions, Suspensions, and Colloids Science, Chemistry ShowMe Is Chocolate Milk A Solution Colloid Or Suspension Understand particles in a solution, and explore solution, suspension, and colloid examples. And freshly stirred paint and hot chocolate (suspensions). Solutions exhibit completely different behavior from suspensions. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.animalia-life.club
Colloid Milk Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. And freshly stirred paint and hot chocolate (suspensions). If you let a suspension sit for long enough, however, the particles will. Examples would be fog and milk (colloids); A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Compare colloids,. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Is Chocolate Milk A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely different behavior from suspensions. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Chapter 8 Solution Chemistry ppt download Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. (c) a suspension, such as mud, is a. Understand particles in a solution, and explore solution, suspension, and colloid examples. And freshly stirred paint and hot chocolate (suspensions). If you let a suspension sit for long enough, however, the particles will. (b) in a colloid, such as milk, the particles are much. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Chapter 2 Matter. ppt download Is Chocolate Milk A Solution Colloid Or Suspension Examples would be fog and milk (colloids); A solution may be colored, but it is transparent, the molecules or ions are. Understand particles in a solution, and explore solution, suspension, and colloid examples. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Both suspensions and colloids are heterogeneous mixtures, but. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Types of Mixtures The saga continues….. ppt download Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. And freshly stirred paint and hot chocolate (suspensions). If you let a suspension sit for long enough, however, the particles will. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.youtube.com
Types of mixtures Colloids YouTube Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. A solution may be colored, but it is transparent, the molecules or ions are. Solutions exhibit completely. Is Chocolate Milk A Solution Colloid Or Suspension.
From slideplayer.com
Elements, Compounds, and Mixtures ppt download Is Chocolate Milk A Solution Colloid Or Suspension And freshly stirred paint and hot chocolate (suspensions). (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Both suspensions and colloids are heterogeneous mixtures, but the particle. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.animalia-life.club
Colloid Milk Is Chocolate Milk A Solution Colloid Or Suspension (c) a suspension, such as mud, is a. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. If. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. And freshly stirred paint and hot chocolate (suspensions). Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A solution may be colored, but it is transparent, the molecules or ions are. Examples would be fog and milk (colloids); A colloid is a heterogeneous mixture in which. Is Chocolate Milk A Solution Colloid Or Suspension.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions Is Chocolate Milk A Solution Colloid Or Suspension If you let a suspension sit for long enough, however, the particles will. Solutions exhibit completely different behavior from suspensions. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Understand particles in a solution, and explore solution, suspension, and colloid examples. A colloid is a heterogeneous mixture in which the dispersed particles are. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.animalia-life.club
Examples Of Colloids Mixtures Is Chocolate Milk A Solution Colloid Or Suspension (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. (c) a. Is Chocolate Milk A Solution Colloid Or Suspension.
From byjus.com
Milk is a colloid. Select the correct statement regarding milk as Is Chocolate Milk A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are. Understand particles in a solution, and explore solution, suspension, and colloid examples. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Solutions exhibit completely different behavior from suspensions. And freshly stirred paint and hot chocolate (suspensions). Compare colloids, suspensions,. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. (c) a suspension, such as mud, is a. Compare colloids, suspensions, solutions, and find their differences. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Examples would be fog and milk (colloids); Understand particles in a solution, and explore solution, suspension, and colloid. Is Chocolate Milk A Solution Colloid Or Suspension.
From www.vrogue.co
What Is A Colloid Definition And Examples vrogue.co Is Chocolate Milk A Solution Colloid Or Suspension Compare colloids, suspensions, solutions, and find their differences. (b) in a colloid, such as milk, the particles are much larger but remain dispersed and do not settle. Understand particles in a solution, and explore solution, suspension, and colloid examples. A solution may be colored, but it is transparent, the molecules or ions are. Examples would be fog and milk (colloids);. Is Chocolate Milk A Solution Colloid Or Suspension.
From sciencenotes.org
What Is a Colloid? Definition and Examples Is Chocolate Milk A Solution Colloid Or Suspension Solutions exhibit completely different behavior from suspensions. Compare colloids, suspensions, solutions, and find their differences. If you let a suspension sit for long enough, however, the particles will. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. And freshly stirred paint and hot chocolate (suspensions). Both. Is Chocolate Milk A Solution Colloid Or Suspension.