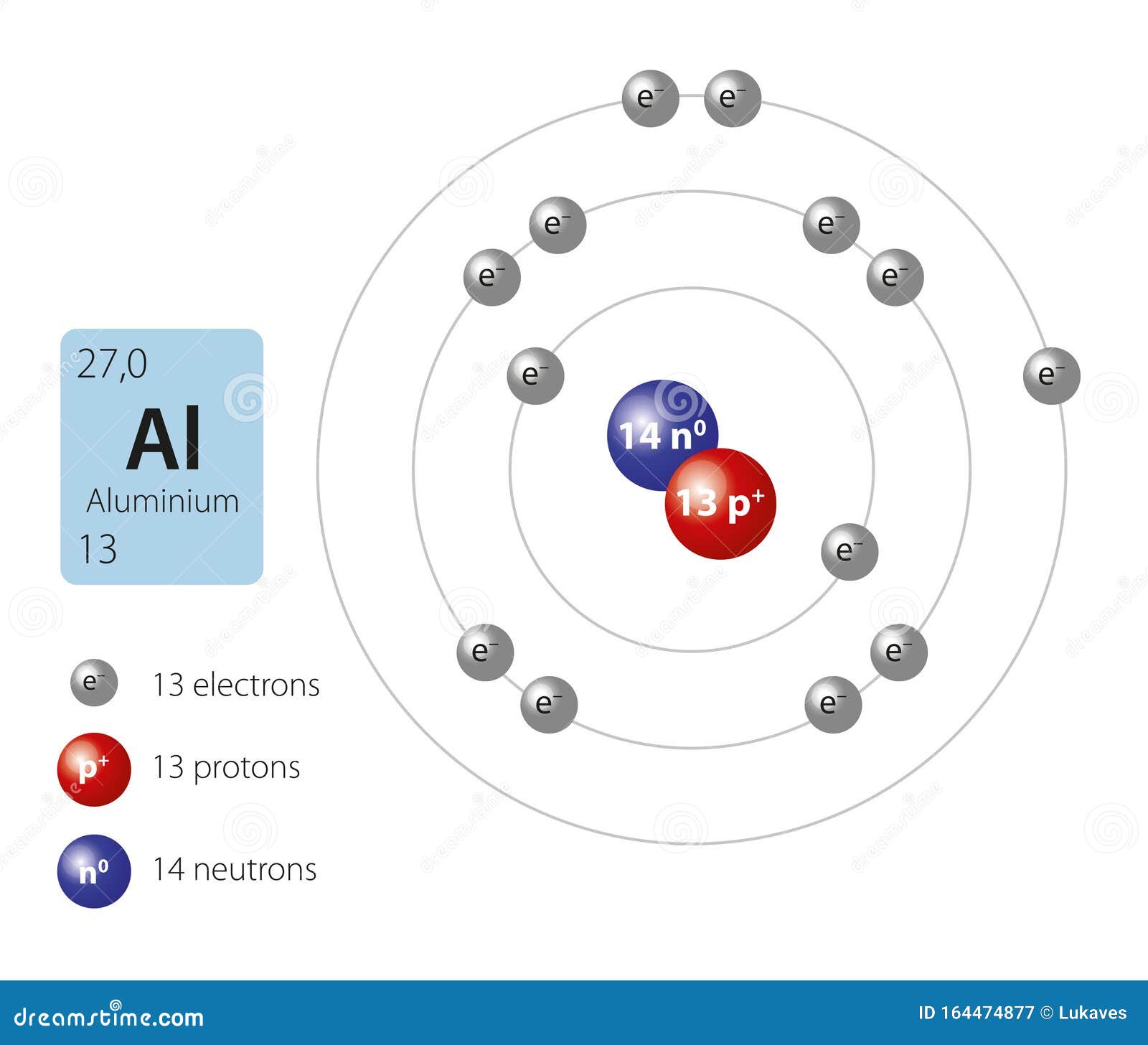
Aluminum Atom Loses 3 Electrons . An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. Ions are formed when atoms gain or lose electrons. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. A cation (positively charged ion) forms when one or more electrons are removed from a. The aluminum atom loses its three valence electrons. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In the formation of al. An alkaline earth metal (group 2) loses.
from www.dreamstime.com
The aluminum atom loses its three valence electrons. An alkaline earth metal (group 2) loses. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. Ions are formed when atoms gain or lose electrons. In the formation of al.
Model of aluminium atom stock vector. Illustration of science 164474877
Aluminum Atom Loses 3 Electrons The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. The aluminum atom loses its three valence electrons. An alkaline earth metal (group 2) loses. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. A cation (positively charged ion) forms when one or more electrons are removed from a. In the formation of al. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Ions are formed when atoms gain or lose electrons.
From www.youtube.com
How to find Protons & Electrons for the Aluminum ion (Al 3+) YouTube Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. Ions are formed when atoms gain or lose electrons. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. In the formation of al. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$),. Aluminum Atom Loses 3 Electrons.
From www.slideserve.com
PPT 18.38 PowerPoint Presentation, free download ID1776567 Aluminum Atom Loses 3 Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are formed when atoms gain or lose electrons. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as. Aluminum Atom Loses 3 Electrons.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. Ions are formed when atoms gain or lose electrons. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. In the formation of al. An atom of aluminum contains 13 protons and 13 electrons, 3 of. Aluminum Atom Loses 3 Electrons.
From www.toppr.com
Aluminium atom loses electrons in successive steps to form Al', Al' and Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. Ions are formed when atoms gain or lose electrons. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. To illustrate, an. Aluminum Atom Loses 3 Electrons.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. Ions are formed when atoms gain or lose electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. The mg 2+ ion,. Aluminum Atom Loses 3 Electrons.
From maribel-bogspotlawson.blogspot.com
How Many Electrons Are in the Outer Shell of Aluminum Aluminum Atom Loses 3 Electrons In the formation of al. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. Ions are formed when atoms gain or lose electrons. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; The aluminum atom loses. Aluminum Atom Loses 3 Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Aluminum (Al) YouTube Aluminum Atom Loses 3 Electrons Ions are formed when atoms gain or lose electrons. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. In the formation of. Aluminum Atom Loses 3 Electrons.
From wirepartfettuccine.z21.web.core.windows.net
Aluminum Electron Dot Diagram Aluminum Atom Loses 3 Electrons Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. A cation (positively charged ion) forms when one or more electrons are removed from a. In the formation of al. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons,. Aluminum Atom Loses 3 Electrons.
From www.numerade.com
SOLVED When aluminum reacts with sulfur to form an ionic compound Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. A cation (positively charged ion) forms when one or more electrons are removed from a. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a. Aluminum Atom Loses 3 Electrons.
From www.museoinclusivo.com
How Many Electrons Does an Aluminum Atom Have? Exploring the Atomic Aluminum Atom Loses 3 Electrons The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. An alkaline earth metal (group 2) loses. The mg 2+ ion,. Aluminum Atom Loses 3 Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Loses 3 Electrons An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. A cation (positively charged ion) forms when one or more electrons are removed from a. Each. Aluminum Atom Loses 3 Electrons.
From www.bartleby.com
Answered Energy Refer to the correctly filled… bartleby Aluminum Atom Loses 3 Electrons Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. The aluminum atom loses its three valence electrons. A cation (positively charged ion). Aluminum Atom Loses 3 Electrons.
From circuitwiringgear.z21.web.core.windows.net
Aluminum Electron Dot Diagram Aluminum Atom Loses 3 Electrons To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged. Aluminum Atom Loses 3 Electrons.
From www.sciencephoto.com
Aluminum, atomic structure Stock Image C018/3694 Science Photo Aluminum Atom Loses 3 Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. In the formation of al. Ions are formed when atoms gain or lose electrons. An atom of aluminum contains 13 protons and 13 electrons, 3 of. Aluminum Atom Loses 3 Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Loses 3 Electrons An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. The aluminum atom loses its three valence electrons. In the formation of al. To illustrate, an atom of an alkali. Aluminum Atom Loses 3 Electrons.
From www.alamy.com
A Aluminium atom diagram Stock Vector Image & Art Alamy Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. An alkaline earth metal (group 2) loses. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. Ions are formed when. Aluminum Atom Loses 3 Electrons.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminum Atom Loses 3 Electrons An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. A cation (positively charged ion) forms when one or more electrons are removed from a. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; The mg 2+. Aluminum Atom Loses 3 Electrons.
From aluminumgenjin.blogspot.com
Aluminum Electron Configuration For Aluminum Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. To illustrate, an atom of an alkali metal (group 1). Aluminum Atom Loses 3 Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Aluminum Atom Loses 3 Electrons Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. The aluminum atom loses its three valence electrons. Atoms that lose electrons acquire. Aluminum Atom Loses 3 Electrons.
From e-eduanswers.com
What is the overall charge of a Aluminum atom that loses 3 electrons Aluminum Atom Loses 3 Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. An alkaline earth. Aluminum Atom Loses 3 Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Atom Loses 3 Electrons An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all. Aluminum Atom Loses 3 Electrons.
From www.slideserve.com
PPT Chapter 5 Chemical Bonding PowerPoint Presentation, free Aluminum Atom Loses 3 Electrons Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In the formation of al. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; An alkaline earth metal (group 2) loses. Ions are formed when atoms gain. Aluminum Atom Loses 3 Electrons.
From www.numerade.com
SOLVED When an aluminum atom (Al) an aluminum ion (Ap+), it Aluminum Atom Loses 3 Electrons To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; Ions are formed when atoms gain or lose electrons. The aluminum atom loses its three valence electrons. In the formation of al. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of. Aluminum Atom Loses 3 Electrons.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. In the formation of al. Ions are formed when atoms gain or lose electrons. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. A. Aluminum Atom Loses 3 Electrons.
From periodictable.me
Aluminum Valence Electrons Aluminum Valency (Al) with Dot Diagram Aluminum Atom Loses 3 Electrons An atom of aluminum contains 13 protons and 13 electrons, 3 of which can be classified as valence electrons, as determined. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. The easiest way to explain it is that $\ce{al}$ has one unpaired electron. Aluminum Atom Loses 3 Electrons.
From www.newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminum Atom Loses 3 Electrons The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. A cation (positively charged ion) forms when one or more electrons are removed from a. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; An. Aluminum Atom Loses 3 Electrons.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. Ions are formed when atoms gain or lose electrons. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. To illustrate,. Aluminum Atom Loses 3 Electrons.
From kdi-ppi.com
The Essential Guide to Understanding the Bohr Diagram of Aluminum Aluminum Atom Loses 3 Electrons The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. In the formation of al. The aluminum atom loses its three valence electrons. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge; An alkaline earth. Aluminum Atom Loses 3 Electrons.
From slideplayer.com
Atomic Structure and The Periodic Table ppt download Aluminum Atom Loses 3 Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are formed when atoms gain or lose electrons. An alkaline earth metal (group 2) loses. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. An atom of aluminum contains 13 protons and 13 electrons,. Aluminum Atom Loses 3 Electrons.
From www.slideserve.com
PPT Ch 7 Review Ionic Compounds PowerPoint Presentation ID6282540 Aluminum Atom Loses 3 Electrons The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$), and $\ce{mg}$'s highest energy. An alkaline earth metal (group 2) loses. In the formation of al. The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Atoms that lose electrons acquire a. Aluminum Atom Loses 3 Electrons.
From byjus.com
Does aluminium require two more electron to complete its octet since it Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. Ions are formed when atoms gain or lose electrons. In the formation of al. The mg 2+ ion, the al 3+ ion, the na + ion,. Aluminum Atom Loses 3 Electrons.
From www.dreamstime.com
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminum Atom Loses 3 Electrons The aluminum atom loses its three valence electrons. An alkaline earth metal (group 2) loses. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In the formation of al. The easiest way to explain it is that $\ce{al}$ has one unpaired electron in it's highest energy orbital ($\mathrm{3p}$),. Aluminum Atom Loses 3 Electrons.
From slideplayer.com
Ionic Bonding. ppt download Aluminum Atom Loses 3 Electrons The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. The aluminum atom loses its three valence electrons. Ions are formed when atoms gain or lose electrons. To illustrate, an. Aluminum Atom Loses 3 Electrons.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminum Atom Loses 3 Electrons The mg 2+ ion, the al 3+ ion, the na + ion, and the ne atom are all isoelectronic. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the positive. In the formation of al. Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation. Aluminum Atom Loses 3 Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Atom Loses 3 Electrons Each neutral aluminum atom loses three electrons to produce an aluminum ion with an oxidation state of +3 in the product, so aluminum has been oxidized. An alkaline earth metal (group 2) loses. The aluminum atom loses its three valence electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance. Aluminum Atom Loses 3 Electrons.