Bromine Chlorine Substitution . this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. while previous position modulations have focused on only one substituent, this study shows the development of three. halogenation of benzene (i.e.
from askanydifference.com
in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. while previous position modulations have focused on only one substituent, this study shows the development of three. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. halogenation of benzene (i.e. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of.
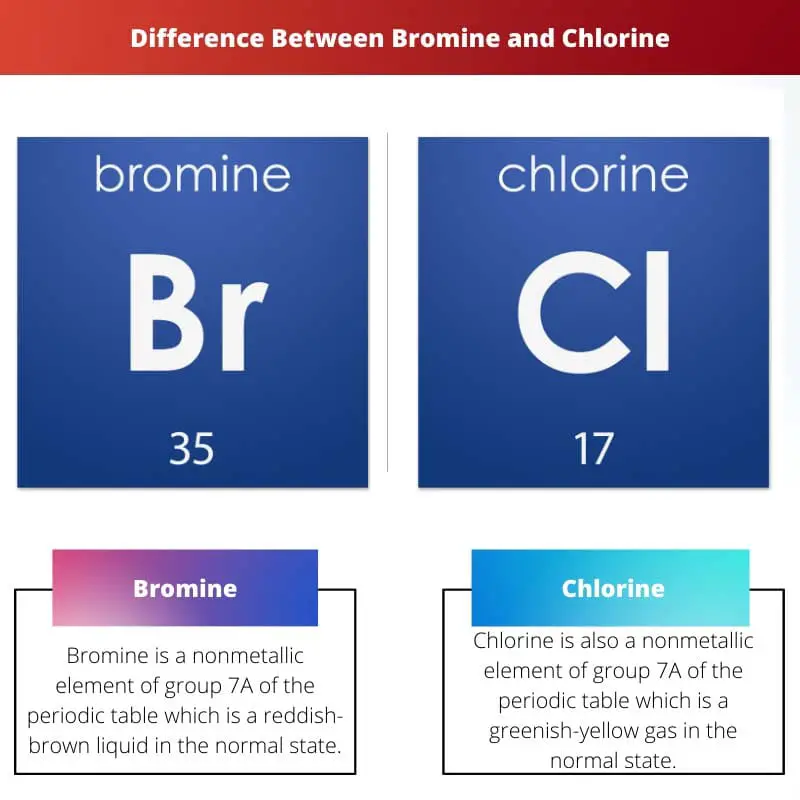
Bromine vs Chlorine Difference and Comparison
Bromine Chlorine Substitution while previous position modulations have focused on only one substituent, this study shows the development of three. while previous position modulations have focused on only one substituent, this study shows the development of three. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. halogenation of benzene (i.e. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic.
From www.numerade.com
SOLVEDWrite equations to illustrate both aromatic and aliphatic substitution reactions of Bromine Chlorine Substitution Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. this page gives you the. Bromine Chlorine Substitution.
From chem.libretexts.org
15.6 Synthetic parallel electrophilic aromatic substitution in the lab Chemistry LibreTexts Bromine Chlorine Substitution while previous position modulations have focused on only one substituent, this study shows the development of three. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. alkyl radicals rapidly react with other molecules. Bromine Chlorine Substitution.
From www.toppr.com
Aryl chlorides and bromides can be easily prepared by elelctrophilic substitution of arenes with Bromine Chlorine Substitution as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. while previous position modulations have focused on only one substituent, this study shows the development of three. alkyl. Bromine Chlorine Substitution.
From www.slideserve.com
PPT α Substitution and Carbonyl Condensation Reactions PowerPoint Presentation ID3004469 Bromine Chlorine Substitution as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. halogenation of benzene (i.e. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. in our laboratory, a model study of the substitution of iodine and bromine for. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Organic Chemistry Bromine Chlorine Substitution alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. this page gives you the facts. Bromine Chlorine Substitution.
From slideplayer.com
Electrophilic Substitution Reactions ppt video online download Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. . Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromination vs. Chlorination Bromine Chlorine Substitution while previous position modulations have focused on only one substituent, this study shows the development of three. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. alkyl radicals rapidly react with other molecules. Bromine Chlorine Substitution.
From www.jove.com
12874.jpg Bromine Chlorine Substitution halogenation of benzene (i.e. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by. Bromine Chlorine Substitution.
From www.numerade.com
SOLVED 5 Phenol adds bromine and chlorine by electrophilic aromatic substitution The reaction Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. while previous position modulations have focused on only one substituent, this study shows the development of three. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. in our laboratory, a model study of the substitution of iodine and. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Chlorine Substitution alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. halogenation of benzene (i.e. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Chlorine Substitution halogenation of benzene (i.e. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. alkyl radicals rapidly react with other molecules or other radicals that provide another electron. Bromine Chlorine Substitution.
From www.nagwa.com
Question Video Predicting the Products of a Substitution Reaction Nagwa Bromine Chlorine Substitution Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. halogenation of benzene (i.e. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. this page gives you the. Bromine Chlorine Substitution.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chemistry LibreTexts Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. halogenation of benzene (i.e. when chloromethane (or methyl chloride). Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. . Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Bromine Chlorine Substitution in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. alkyl radicals rapidly react with other molecules or other radicals that. Bromine Chlorine Substitution.
From www.toppr.com
(b) By electrophilie substitution Aryl chlorides and bromides can be easily prepared by Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. alkyl radicals rapidly react with other molecules or other radicals that. Bromine Chlorine Substitution.
From www.numerade.com
SOLVED Write the equations for the substitution reaction of methane with bromine under the Bromine Chlorine Substitution alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. halogenation of benzene (i.e. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. while previous position modulations have focused on only one substituent, this study shows the development of three. this page gives you the facts and. Bromine Chlorine Substitution.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. when chloromethane (or methyl chloride) reacts with cl 2, another. Bromine Chlorine Substitution.
From www.researchgate.net
Analytical scheme for radiochemical purification of chlorine, bromine... Download Scientific Bromine Chlorine Substitution halogenation of benzene (i.e. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Chlorine Substitution in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Selectivity in Free Radical Reactions Bromine vs. Chlorine — Master Organic Chemistry Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. halogenation of benzene (i.e. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by. Bromine Chlorine Substitution.
From www.doheny.com
Bromine vs. Chlorine Everything You Need to Know Bromine Chlorine Substitution in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. . Bromine Chlorine Substitution.
From www.researchgate.net
(PDF) Altering the Positions of Chlorine and Bromine Substitution on the End Group Enables High Bromine Chlorine Substitution while previous position modulations have focused on only one substituent, this study shows the development of three. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. as seen in the first example below,. Bromine Chlorine Substitution.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points, Definitions, Examples Bromine Chlorine Substitution as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. . Bromine Chlorine Substitution.
From www.chemistrysteps.com
SOCl2 and PBr3 Chemistry Steps Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. halogenation of benzene (i.e. this page gives you the. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Chlorine Substitution Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. alkyl radicals rapidly react with other molecules or other radicals. Bromine Chlorine Substitution.
From thecontentauthority.com
Bromine vs Chlorine Do These Mean The Same? How To Use Them Bromine Chlorine Substitution alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. in our laboratory, a model study of the substitution of. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Introduction to Free Radical Substitution Reactions Master Organic Chemistry Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. halogenation of benzene (i.e. in our laboratory, a model. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Chlorine Substitution this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. halogenation of benzene (i.e. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by. Bromine Chlorine Substitution.
From www.youtube.com
Aromatic Halogenation Mechanism Chlorination, Iodination & Bromination of Benzene YouTube Bromine Chlorine Substitution as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. while previous position modulations have focused on only one substituent, this study shows the development of three. halogenation of benzene (i.e. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in. Bromine Chlorine Substitution.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. while previous position modulations have focused on only one substituent, this study shows the development of three. halogenation of benzene (i.e. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below,. Bromine Chlorine Substitution.
From www.slideserve.com
PPT CCEA organic reaction mechanisms PowerPoint Presentation, free download ID5949384 Bromine Chlorine Substitution halogenation of benzene (i.e. alkyl radicals rapidly react with other molecules or other radicals that provide another electron to form a chemical bond. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. as seen in the first example below, one of the hydrogens on ethane is replaced by an atom of. in our laboratory, a model. Bromine Chlorine Substitution.
From www.differencebetween.com
Difference Between Bromine and Chlorine Compare the Difference Between Similar Terms Bromine Chlorine Substitution while previous position modulations have focused on only one substituent, this study shows the development of three. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. halogenation of benzene (i.e. this page gives you the facts and a simple, uncluttered mechanism for the free radical substitution reaction between methane. alkyl radicals rapidly react with other molecules. Bromine Chlorine Substitution.
From www.numerade.com
SOLVED 5) Phenol adds bromine ant chlorine by electrophllic aromatie substitution; The reaction Bromine Chlorine Substitution halogenation of benzene (i.e. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. while previous position modulations have focused. Bromine Chlorine Substitution.
From www.docbrown.info
Free radical reaction of alkanes with bromine chlorine conditions products uses mechanisms Bromine Chlorine Substitution when chloromethane (or methyl chloride) reacts with cl 2, another hydrogen is replaced by a chlorine atom to give. while previous position modulations have focused on only one substituent, this study shows the development of three. in our laboratory, a model study of the substitution of iodine and bromine for chlorine in the simplest. Chlorination, iodination, and. Bromine Chlorine Substitution.