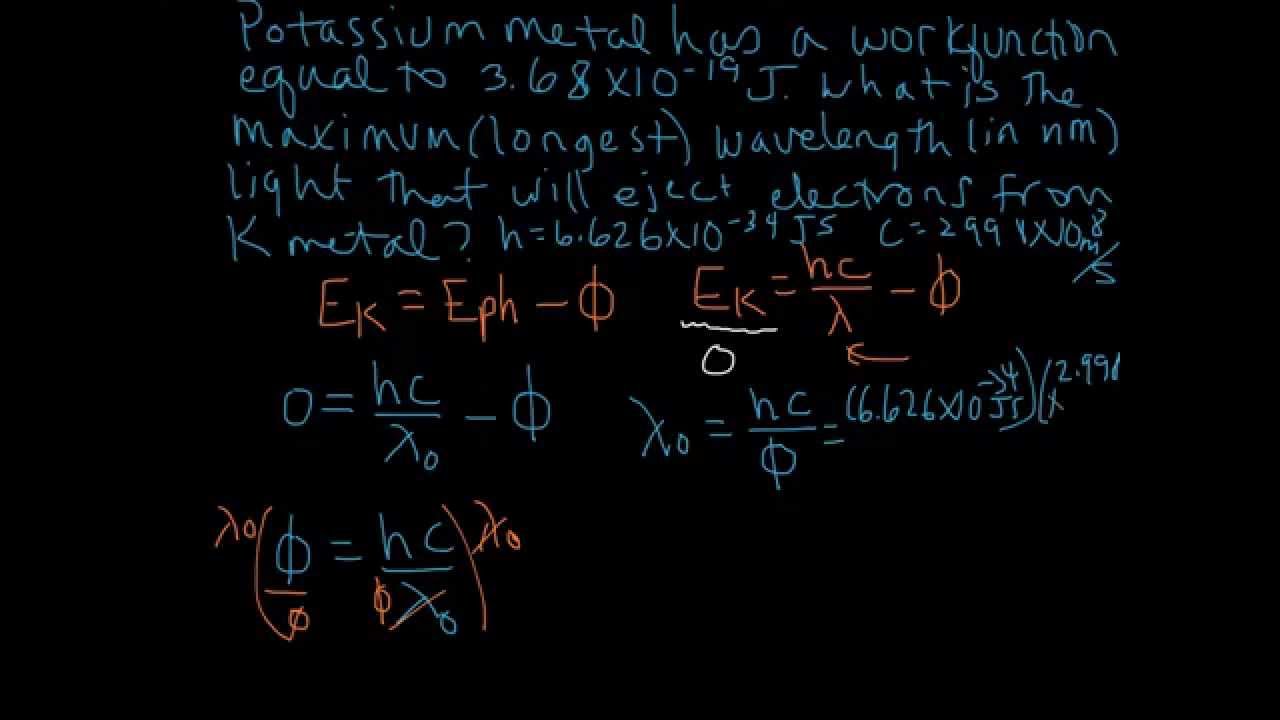Threshold Energy Calculation Formula . Min = k th = −q. \ [ et = h \times tr \] where: \ (et\) is the energy threshold in. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The work function φ, or threshold energy, of a material, is defined as: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Consider the electrons in a metal as. The minimum energy required to release a photoelectron from the surface of a metal. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The formula to calculate the energy threshold is: This equation is valid if the energies are much less than rest mass energies of the involved particles.
from www.youtube.com
\ (et\) is the energy threshold in. This equation is valid if the energies are much less than rest mass energies of the involved particles. Min = k th = −q. Consider the electrons in a metal as. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The work function φ, or threshold energy, of a material, is defined as: The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. \ [ et = h \times tr \] where: The minimum energy required to release a photoelectron from the surface of a metal. The formula to calculate the energy threshold is:
Calculation of Threshold Wavelength in Photoelectric Effect YouTube
Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. Min = k th = −q. The work function φ, or threshold energy, of a material, is defined as: Consider the electrons in a metal as. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. \ [ et = h \times tr \] where: The minimum energy required to release a photoelectron from the surface of a metal. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. This equation is valid if the energies are much less than rest mass energies of the involved particles. The formula to calculate the energy threshold is: \ (et\) is the energy threshold in.
From slideplayer.com
Wave / Particle Duality ppt download Threshold Energy Calculation Formula Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Consider the electrons in a metal as. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state.. Threshold Energy Calculation Formula.
From thefactfactor.com
Stopping potential, Photoelectric work function, maximum energy Threshold Energy Calculation Formula \ [ et = h \times tr \] where: Min = k th = −q. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the. Threshold Energy Calculation Formula.
From www.youtube.com
(English) The threshold frequency v0 for a metal is 7.0 X 10^14 s1 Threshold Energy Calculation Formula The minimum energy required to release a photoelectron from the surface of a metal. \ (et\) is the energy threshold in. This equation is valid if the energies are much less than rest mass energies of the involved particles. The formula to calculate the energy threshold is: Threshold energy= average of the initial kinetic energy possessed by the reactants +. Threshold Energy Calculation Formula.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy Calculation Formula The minimum energy required to release a photoelectron from the surface of a metal. \ (et\) is the energy threshold in. Min = k th = −q. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. This equation is valid if the. Threshold Energy Calculation Formula.
From www.chegg.com
Solved Vo Threshold Voltage What is threshold voltage Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. The minimum energy required to release a photoelectron from the surface of a metal. Min = k th = −q. \ (et\) is the energy threshold in. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the. Threshold Energy Calculation Formula.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Calculation Formula The work function φ, or threshold energy, of a material, is defined as: The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The formula to calculate the energy threshold is: The minimum energy required to release a photoelectron from the. Threshold Energy Calculation Formula.
From www.youtube.com
Calculate threshold frequency Dual nature of light Physics Khan Threshold Energy Calculation Formula The formula to calculate the energy threshold is: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. This equation is valid if the energies are much less than rest mass energies of the involved particles. The activation energy (\ (e_a\)), labeled \. Threshold Energy Calculation Formula.
From www.researchgate.net
How to calculate the laser power density and laser energy density Threshold Energy Calculation Formula The minimum energy required to release a photoelectron from the surface of a metal. Consider the electrons in a metal as. This equation is valid if the energies are much less than rest mass energies of the involved particles. \ (et\) is the energy threshold in. The work function φ, or threshold energy, of a material, is defined as: To. Threshold Energy Calculation Formula.
From www.animalia-life.club
Law Of Conservation Of Energy Equation Chemistry Threshold Energy Calculation Formula Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. Consider the electrons in a metal as. \ (et\) is the energy threshold in. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function. Threshold Energy Calculation Formula.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical Threshold Energy Calculation Formula \ (et\) is the energy threshold in. This equation is valid if the energies are much less than rest mass energies of the involved particles. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The work function φ, or threshold energy, of. Threshold Energy Calculation Formula.
From www.researchgate.net
The threshold energy E 0 is determined by the equation (3.1). The red Threshold Energy Calculation Formula The minimum energy required to release a photoelectron from the surface of a metal. The formula to calculate the energy threshold is: Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. To find the threshold is a naive way, we could calculate the velocity of. Threshold Energy Calculation Formula.
From electronics.stackexchange.com
transistors Calculating CMOS threshold voltage Electrical Threshold Energy Calculation Formula The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The work function φ, or threshold energy, of a material, is defined as: This equation is valid if the energies are much less than rest mass energies of the involved particles.. Threshold Energy Calculation Formula.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy Calculation Formula The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a metal. Min = k th = −q. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as. Threshold Energy Calculation Formula.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Calculation Formula Min = k th = −q. \ [ et = h \times tr \] where: \ (et\) is the energy threshold in. The formula to calculate the energy threshold is: The work function φ, or threshold energy, of a material, is defined as: This equation is valid if the energies are much less than rest mass energies of the involved. Threshold Energy Calculation Formula.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. Min = k th = −q. The minimum energy required to release a photoelectron from the surface of a metal. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the. Threshold Energy Calculation Formula.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Calculation Formula Consider the electrons in a metal as. Min = k th = −q. The minimum energy required to release a photoelectron from the surface of a metal. \ [ et = h \times tr \] where: This equation is valid if the energies are much less than rest mass energies of the involved particles. Threshold energy= average of the initial. Threshold Energy Calculation Formula.
From www.linstitute.net
AQA A Level Physics复习笔记2.4.3 The Photoelectric Equation翰林国际教育 Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. The work function φ, or threshold energy, of a material, is defined as: Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. To find the threshold is. Threshold Energy Calculation Formula.
From howtova.blogspot.com
How To Calculate Threshold Frequency HOWTOVA Threshold Energy Calculation Formula To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. \ (et\) is the energy threshold in. Consider the electrons in a metal as. Min = k th = −q. The minimum energy required to release a photoelectron from the surface of a. Threshold Energy Calculation Formula.
From www.tessshebaylo.com
Binding Energy Equation Example Tessshebaylo Threshold Energy Calculation Formula Consider the electrons in a metal as. The formula to calculate the energy threshold is: Min = k th = −q. \ (et\) is the energy threshold in. The minimum energy required to release a photoelectron from the surface of a metal. \ [ et = h \times tr \] where: This equation is valid if the energies are much. Threshold Energy Calculation Formula.
From www.youtube.com
C.7 Calculating energy released in nuclear reactions (HL) YouTube Threshold Energy Calculation Formula Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. Min = k th = −q. This. Threshold Energy Calculation Formula.
From byjus.com
select the incorrect statement (1) The minimum amount of energy Threshold Energy Calculation Formula Consider the electrons in a metal as. \ (et\) is the energy threshold in. \ [ et = h \times tr \] where: This equation is valid if the energies are much less than rest mass energies of the involved particles. The minimum energy required to release a photoelectron from the surface of a metal. Threshold energy= average of the. Threshold Energy Calculation Formula.
From www.mdpi.com
Electronics Free FullText A Threshold Voltage Model for AOS TFTs Threshold Energy Calculation Formula The work function φ, or threshold energy, of a material, is defined as: \ [ et = h \times tr \] where: To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Consider the electrons in a metal as. Min = k th. Threshold Energy Calculation Formula.
From www.youtube.com
Calculation of Threshold Wavelength in Photoelectric Effect YouTube Threshold Energy Calculation Formula Min = k th = −q. \ [ et = h \times tr \] where: The formula to calculate the energy threshold is: \ (et\) is the energy threshold in. The minimum energy required to release a photoelectron from the surface of a metal. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy. Threshold Energy Calculation Formula.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy Calculation Formula Min = k th = −q. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The work function φ, or threshold energy, of a material, is defined as: \ (et\) is the energy threshold in. The activation energy (\ (e_a\)), labeled \. Threshold Energy Calculation Formula.
From www.slideserve.com
PPT CHEM 312 Lecture 9 Nuclear Reactions PowerPoint Presentation Threshold Energy Calculation Formula Min = k th = −q. The formula to calculate the energy threshold is: Consider the electrons in a metal as. The minimum energy required to release a photoelectron from the surface of a metal. To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam. Threshold Energy Calculation Formula.
From nanohub.org
Resources [Illinois] PHYS466 2013 Lecture 28 PIMC Intro Threshold Energy Calculation Formula The work function φ, or threshold energy, of a material, is defined as: The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. Consider the electrons in a metal as. Min = k th = −q. The minimum energy required to. Threshold Energy Calculation Formula.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. \ (et\) is the energy threshold in. \ [ et = h \times tr \] where: The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a. Threshold Energy Calculation Formula.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Calculation Formula To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. The minimum energy required to release a photoelectron from the surface of a metal. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the. Threshold Energy Calculation Formula.
From www.youtube.com
13.18 How to calculate the energy released in a nuclear reaction YouTube Threshold Energy Calculation Formula The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. Min = k th = −q. \ [ et = h \times tr \] where: Consider the electrons in a metal as. This equation is valid if the energies are much. Threshold Energy Calculation Formula.
From www.meritnation.com
The threshold frequency v0 for a metal is 5 0 *10 ^14s what will be the Threshold Energy Calculation Formula This equation is valid if the energies are much less than rest mass energies of the involved particles. The formula to calculate the energy threshold is: \ (et\) is the energy threshold in. Min = k th = −q. Consider the electrons in a metal as. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is. Threshold Energy Calculation Formula.
From scoop.eduncle.com
What is the formula to find q value and threshold energy of a nuclear Threshold Energy Calculation Formula To find the threshold is a naive way, we could calculate the velocity of the center of momentum frame as a function of the beam energy, then. Min = k th = −q. The minimum energy required to release a photoelectron from the surface of a metal. The work function φ, or threshold energy, of a material, is defined as:. Threshold Energy Calculation Formula.
From www.youtube.com
A metal has a threshold wavelength fo `6000 Å`. Calculate (i) threshold Threshold Energy Calculation Formula The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a metal. \ (et\) is the energy threshold in. Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. The formula. Threshold Energy Calculation Formula.
From www.diplomageeks.com
Define Threshold frequency,Threshold wavelength,Work function, Stopping Threshold Energy Calculation Formula \ [ et = h \times tr \] where: Consider the electrons in a metal as. The work function φ, or threshold energy, of a material, is defined as: The minimum energy required to release a photoelectron from the surface of a metal. This equation is valid if the energies are much less than rest mass energies of the involved. Threshold Energy Calculation Formula.
From first10em.com
FullThresholdcalculation First10EM Threshold Energy Calculation Formula The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between the reactants and the activated complex, also known as transition state. The work function φ, or threshold energy, of a material, is defined as: Consider the electrons in a metal as. \ (et\) is the energy threshold in. Min = k th. Threshold Energy Calculation Formula.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Calculation Formula Threshold energy= average of the initial kinetic energy possessed by the reactants + activation energy (ea) if the reactants at the start of the. This equation is valid if the energies are much less than rest mass energies of the involved particles. The activation energy (\ (e_a\)), labeled \ (\delta {g^ {\ddagger}}\) in figure 2, is the energy difference between. Threshold Energy Calculation Formula.