Copper Sulfate Ph . Melting point 200 °c with decomposition. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Find out how to prepare,. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9, and 2.53 × 10 −8,. Find out the reactions, standard potentials, physical changes and ph. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. However, as quantified in ce4501. My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Copper (ii) sulfate is a metal. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses.
from animalia-life.club
However, as quantified in ce4501. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Copper (ii) sulfate is a metal. Melting point 200 °c with decomposition. My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Find out how to prepare,. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula.
Anhydrous Cuso4 Coloring Pages
Copper Sulfate Ph Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. However, as quantified in ce4501. Find out the reactions, standard potentials, physical changes and ph. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9, and 2.53 × 10 −8,. My base ph of the cuso4 is about 4.0 or 4.1. Find out how to prepare,. Copper (ii) sulfate is a metal. Melting point 200 °c with decomposition. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses.
From animalia-life.club
Anhydrous Cuso4 Coloring Pages Copper Sulfate Ph Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Melting point 200 °c with decomposition. Copper (ii) sulfate is a metal. My base ph of the cuso4 is about 4.0 or 4.1. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Therefore,. Copper Sulfate Ph.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Copper (ii) sulfate is a metal. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9,. Copper Sulfate Ph.
From www.suchemind.com
CopperSulphateCopperSulfate Copper Sulfate Ph My base ph of the cuso4 is about 4.0 or 4.1. Find out the reactions, standard potentials, physical changes and ph. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the. Copper Sulfate Ph.
From www.researchgate.net
Absorbance spectra of copper sulfate solutions at pH 10.7 and 12.3 Copper Sulfate Ph My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. However, as quantified in ce4501. Copper (ii) sulfate is a metal. Find out the reactions, standard potentials, physical changes and ph. Melting point 200 °c with decomposition. Find out how to. Copper Sulfate Ph.
From www.walmart.com
Cupric Sulfate, Pentahydrate, Laboratory Grade, 500 G Copper Sulfate Ph Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Find out how to prepare,. Melting point 200 °c with decomposition. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. My base ph of the cuso4 is about 4.0 or 4.1. Find out the. Copper Sulfate Ph.
From www.semanticscholar.org
Figure 4 from Effect of pH, concentration and temperature on copper and Copper Sulfate Ph Melting point 200 °c with decomposition. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,.. Copper Sulfate Ph.
From www.aliexpress.com
The new SE 1 ultrafine copper sulfate reference electrode copper Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Find out how to prepare,. However, as quantified in ce4501. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10,. Copper Sulfate Ph.
From www.flinnsci.com
Copper(II) Sulfate, Powder, Lab Grade, 500 g Flinn Scientific Copper Sulfate Ph Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Copper (ii) sulfate is a metal. However, as quantified in ce4501. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Lead oxalate (pbc 2 o 4), lead iodide (pbi. Copper Sulfate Ph.
From www.reddit.com
Copper Sulfate Crystals r/chemistry Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. However, as quantified in ce4501. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9, and. Copper Sulfate Ph.
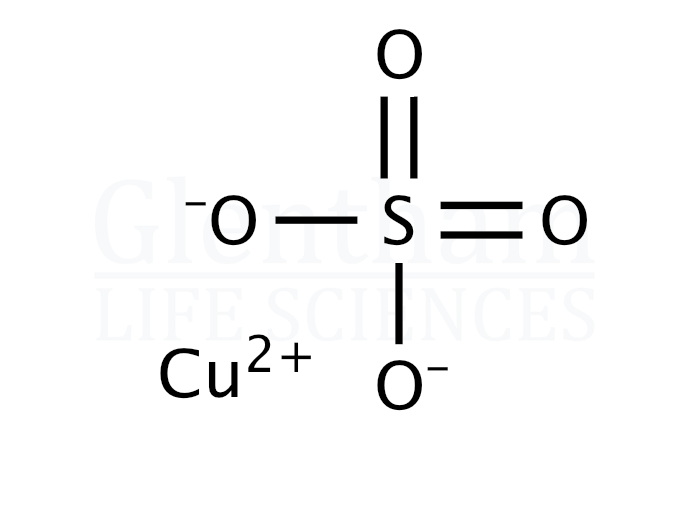
From www.glentham.com
Copper(II) sulfate pentahydrate, 99, Ph. Eur. grade (CAS 7758998 Copper Sulfate Ph Find out the reactions, standard potentials, physical changes and ph. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Copper (ii) sulfate is a metal. Therefore, the contribution of sulfate alone is to. Copper Sulfate Ph.
From www.omniamfg.com
How and When to Perform a Copper Sulfate Test — Omnia MFG Copper Sulfate Ph My base ph of the cuso4 is about 4.0 or 4.1. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. I've tried making a ph 5 and ph 6 buffer solution then adding it. Copper Sulfate Ph.
From www.drugs.com
Cupric Sulfate Injection FDA prescribing information, side effects Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Copper (ii) sulfate is a metal. Find out the reactions, standard potentials, physical changes and ph. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. However, as quantified in ce4501.. Copper Sulfate Ph.
From www.aces.edu
Total Alkalinity Important Before Copper Sulfate Treatments Alabama Copper Sulfate Ph Melting point 200 °c with decomposition. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Find out how to prepare,. My base ph of the cuso4 is about 4.0. Copper Sulfate Ph.
From www.researchgate.net
SEM images of copper clusters deposited from a 50 mM copper sulfate pH Copper Sulfate Ph My base ph of the cuso4 is about 4.0 or 4.1. However, as quantified in ce4501. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Melting point 200 °c with decomposition. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Find out. Copper Sulfate Ph.
From hippocampus.ee
Copper Sulphate pentahydrate feed grade worldwide supplier Copper Sulfate Ph Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Find out how to prepare,. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. I've tried making a ph 5 and ph 6 buffer solution then adding it to. Copper Sulfate Ph.
From pastorres.weebly.com
Copper sulfate pastorres Copper Sulfate Ph Find out how to prepare,. Copper (ii) sulfate is a metal. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. However, as quantified in ce4501. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 ×. Copper Sulfate Ph.
From www.researchgate.net
ait curves of different step potentials at pH 4.5 in copper sulfate Copper Sulfate Ph Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Melting point 200 °c with decomposition. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Therefore, the contribution of sulfate alone is to make the solution slightly more basic. Copper Sulfate Ph.
From ar.inspiredpencil.com
Copper Sulfate Crystals Copper Sulfate Ph However, as quantified in ce4501. Find out how to prepare,. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Melting point 200 °c with decomposition. Copper (ii) sulfate is a metal. My base. Copper Sulfate Ph.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Melting point 200 °c with decomposition. Find out how to prepare,. Copper (ii) sulfate is a metal. Therefore, the contribution. Copper Sulfate Ph.
From imbros.com.au
Copper (II) Sulfate Pentahydrate For Sale Imbros Copper Sulfate Ph Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Find out how to prepare,. Copper (ii) sulfate is a metal. However, as quantified in ce4501. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. My base ph of the cuso4 is about 4.0. Copper Sulfate Ph.
From www.chegg.com
Solved 10 100 8 6 Solubility of Copper Sulfate Copper Sulfate Ph I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9, and 2.53 × 10 −8,. Melting point 200 °c with. Copper Sulfate Ph.
From www.researchgate.net
Temperature dependence of the solubility of copper sulfate in water Copper Sulfate Ph Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Melting point 200 °c with decomposition. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Learn. Copper Sulfate Ph.
From owlcation.com
How to Make a Copper Sulphate Solution? Owlcation Copper Sulfate Ph Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Find out how to prepare,. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are. Copper Sulfate Ph.
From labbox.eu
Copper (II) sulfate pentahydrate EPR Ph. Eur., USP Labbox Export Copper Sulfate Ph I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. However, as quantified in ce4501. Find out how to prepare,. Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9,. Copper Sulfate Ph.
From sciencekitstore.com
Copper Sulfate, Pentahydrate High Purity Chemical for Diverse Copper Sulfate Ph Find out how to prepare,. My base ph of the cuso4 is about 4.0 or 4.1. Copper (ii) sulfate is a metal. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. I've tried making a ph 5 and ph 6 buffer solution then adding it to the. Copper Sulfate Ph.
From www.researchgate.net
Absorbance spectra of the dye/copper sulfate system at pH 10.7 and Copper Sulfate Ph Lead oxalate (pbc 2 o 4), lead iodide (pbi 2), and lead sulfate (pbso 4) are all rather insoluble, with k sp values of 4.8 × 10 −10, 9.8 × 10 −9, and 2.53 × 10 −8,. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Melting. Copper Sulfate Ph.
From shopee.ph
Copper Sulfate 500g AR Laboratory Analytical Reagent ACS Grade Shopee Copper Sulfate Ph Find out how to prepare,. Melting point 200 °c with decomposition. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Find out the reactions, standard potentials, physical changes and ph. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Lead oxalate (pbc. Copper Sulfate Ph.
From sciencekitstore.com
Copper Sulfate, Pentahydrate High Purity Chemical for Diverse Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. However, as quantified in ce4501. Copper (ii) sulfate is. Copper Sulfate Ph.
From auschemsource.com.au
Buy Copper Sulphate Online AUS CHEM SOURCE PTY LTD Copper Sulfate Ph Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Find out the reactions, standard potentials, physical changes and ph. Find out how to prepare,. However, as quantified in ce4501. My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper. Copper Sulfate Ph.
From chemicaldb.netlify.app
Copper ii sulfate solubility Copper Sulfate Ph Melting point 200 °c with decomposition. My base ph of the cuso4 is about 4.0 or 4.1. Copper (ii) sulfate is a metal. Find out how to prepare,. I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its. Copper Sulfate Ph.
From www.sciencephoto.com
Copper Sulphate Stock Image C028/1275 Science Photo Library Copper Sulfate Ph I've tried making a ph 5 and ph 6 buffer solution then adding it to the cuso4 solution,. Melting point 200 °c with decomposition. Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. Copper (ii) sulfate is a metal. Find out how to prepare,. My base ph of the cuso4 is about. Copper Sulfate Ph.
From www.researchgate.net
Soluble copper as a function of pH as measured by filtration through a Copper Sulfate Ph However, as quantified in ce4501. Melting point 200 °c with decomposition. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Find out how to prepare,. Find out the reactions, standard potentials, physical changes and ph. Copper (ii) sulfate is a metal. I've tried making a ph 5 and ph 6 buffer. Copper Sulfate Ph.
From chemicaldb.netlify.app
Copper ii sulfate solubility Copper Sulfate Ph However, as quantified in ce4501. Copper (ii) sulfate is a metal. Learn about the chemical formula, physical and chemical properties, uses, and hazards of copper (ii) sulfate, a blue compound with the formula. Melting point 200 °c with decomposition. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. My base ph. Copper Sulfate Ph.
From www.tradeindia.com
Cuso4 Soluble 4.0 Ph Odourless Copper Sulphate Powder For Industrial Copper Sulfate Ph Therefore, the contribution of sulfate alone is to make the solution slightly more basic than ph 7. However, as quantified in ce4501. Find out the reactions, standard potentials, physical changes and ph. Find out how to prepare,. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Melting point 200 °c with. Copper Sulfate Ph.
From crystalverse.com
The Best Way to Grow Big Copper Sulfate Crystals Crystalverse Copper Sulfate Ph My base ph of the cuso4 is about 4.0 or 4.1. Learn how to perform electrolysis of copper sulfate solution with different types of electrodes and observe the products. Learn about the chemical compound cuso4, also known as copper (ii) sulfate, and its structure, properties, and uses. Copper (ii) sulfate is a metal. Lead oxalate (pbc 2 o 4), lead. Copper Sulfate Ph.