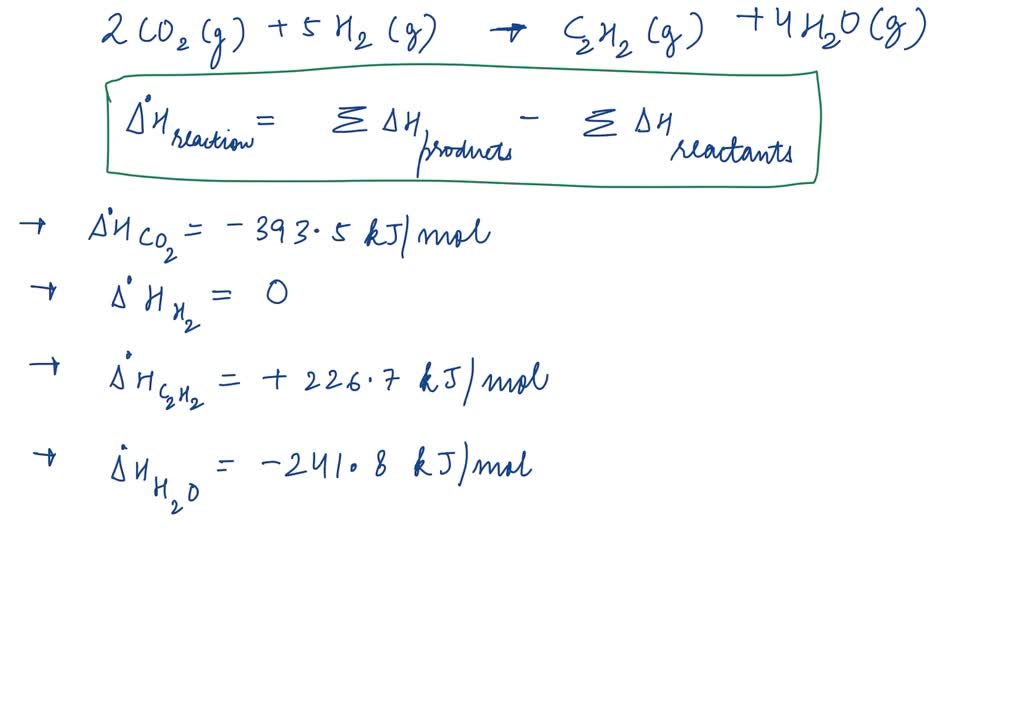
Standard Enthalpy Of Formation Reaction Examples . A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. A formation equation is written. The nought superscript means standard state. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
from www.numerade.com
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The nought superscript means standard state. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A formation equation is written.
SOLVED 'The following problems deal with standard enthalpy of
Standard Enthalpy Of Formation Reaction Examples The standard enthalpy (heat) of reaction is given by δh o rxn. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A formation equation is written. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The nought superscript means standard state. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Reaction Examples The nought superscript means standard state. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. Standard enthalpy of formation (or heat of formation), δhof ,. Standard Enthalpy Of Formation Reaction Examples.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The nought superscript means standard state. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The standard enthalpy (heat) of reaction is given by. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Enthalpy Of Formation Reaction Examples 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The nought superscript means standard state. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy. Standard Enthalpy Of Formation Reaction Examples.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy (heat) of reaction is. Standard Enthalpy Of Formation Reaction Examples.
From www.chegg.com
Solved Example Enthalpy Changes for a Reaction Calculate 4, Standard Enthalpy Of Formation Reaction Examples A formation equation is written. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The standard enthalpy (heat) of reaction is given by δh o rxn. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Enthalpy Of Formation Reaction Examples.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Standard Enthalpy Of Formation Reaction Examples A formation equation is written. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Standard Enthalpy Of Formation Reaction Examples 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Reaction Examples The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A formation equation is written. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with. Standard Enthalpy Of Formation Reaction Examples.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Reaction Examples A formation equation is written. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard. Standard Enthalpy Of Formation Reaction Examples.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when. Standard Enthalpy Of Formation Reaction Examples.
From nasirghopbuck.blogspot.com
How to Calculate the Standard Enthalpy of Combustion Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A formation equation is written. The nought superscript means standard state. The standard enthalpy (heat) of. Standard Enthalpy Of Formation Reaction Examples.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy (heat) of reaction is given by δh o rxn. Use. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The nought superscript means standard state. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A formation equation is written. A. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Reaction Examples A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation ID2308925 Standard Enthalpy Of Formation Reaction Examples A formation equation is written. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Reaction Examples.
From www.chegg.com
Solved The standard enthalpy (or heat) of reaction, delta H, Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. A formation equation is written. Use standard enthalpies of. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Enthalpy Of Formation Reaction Examples 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The nought superscript means standard state. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. A formation equation is written. The standard enthalpy of. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Enthalpies of Formation and Reaction PowerPoint Presentation Standard Enthalpy Of Formation Reaction Examples The standard enthalpy (heat) of reaction is given by δh o rxn. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Reaction Examples 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Reaction Examples The nought superscript means standard state. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The standard enthalpy (heat) of reaction is given by δh o rxn. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Reaction Examples The standard enthalpy (heat) of reaction is given by δh o rxn. Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The nought superscript means standard state. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation Reaction Examples.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The nought superscript means standard state. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy. Standard Enthalpy Of Formation Reaction Examples.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Reaction Examples Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. A formation equation is written. The nought superscript means standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Reaction Examples.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A formation equation is written. The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of reaction \(\delta{h_{rxn}^o}\) is the enthalpy change that occurs when a reaction is carried out with all reactants and products. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The standard enthalpy (heat) of reaction is given by δh o rxn. The nought superscript means standard state. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Standard Enthalpy Of Formation Reaction Examples.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. The nought superscript means standard state. The standard enthalpy (heat) of reaction is given by δh o rxn. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Ch. 15 Energy and Chemical Change PowerPoint Presentation, free Standard Enthalpy Of Formation Reaction Examples The nought superscript means standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy of formation (or heat of formation), δhof , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Reaction Examples Use standard enthalpies of formation to calculate the enthalpy change (in kj) for the reduction of iron(iii) oxide to iron at 298. A formation equation is written. The standard enthalpy (heat) of reaction is given by δh o rxn. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure.. Standard Enthalpy Of Formation Reaction Examples.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. A standard enthalpy of formation \(δh^\circ_\ce{f}\) is an enthalpy change for a reaction in which exactly 1 mole of a pure. The standard enthalpy (heat) of reaction is given by δh o rxn. The nought superscript. Standard Enthalpy Of Formation Reaction Examples.
From www.youtube.com
Standard enthalpy of formation example question YouTube Standard Enthalpy Of Formation Reaction Examples The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The nought superscript means standard state. Use standard enthalpies of formation to calculate. Standard Enthalpy Of Formation Reaction Examples.