What Is The Number Electron Shells . Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The rule to calculate the number of electrons that each shell can hold is 2n 2. Each allowed electron orbit is assigned. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. In the diagram above the energy levels are depicted as the rings around the. Different shells can hold different maximum numbers of electrons. Electrons in the same shell have the same amount of energy. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. The first shell is 2 (1) 2 which gives you 2 electrons. Electron shells and the bohr model figure 1. The general rule stating the number of electrons present in a shell is 2n 2,.
from www.nagwa.com
Each allowed electron orbit is assigned. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The general rule stating the number of electrons present in a shell is 2n 2,. Electron shells and the bohr model figure 1. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. In the diagram above the energy levels are depicted as the rings around the. Different shells can hold different maximum numbers of electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons.
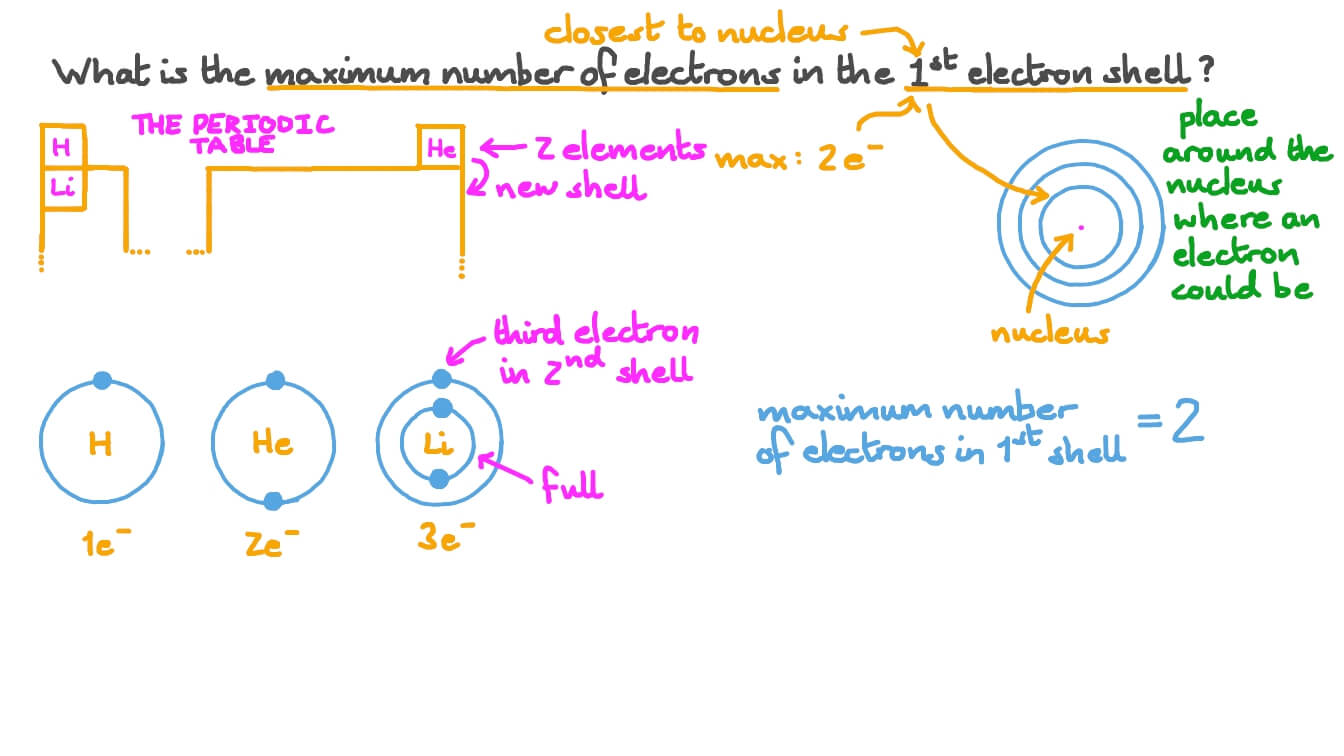
Question Video Identifying the Maximum Number of Electrons in the 1st
What Is The Number Electron Shells Electron shells and the bohr model figure 1. The general rule stating the number of electrons present in a shell is 2n 2,. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electrons in the same shell have the same amount of energy. In the diagram above the energy levels are depicted as the rings around the. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electron shells and the bohr model figure 1. The first shell is 2 (1) 2 which gives you 2 electrons. Different shells can hold different maximum numbers of electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. Each allowed electron orbit is assigned. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons.
From www.numerade.com
SOLVEDWhat is the maximum number of electrons that can occupy the n=4 What Is The Number Electron Shells Electron shells and the bohr model figure 1. Each allowed electron orbit is assigned. The first shell is 2 (1) 2 which gives you 2 electrons. Different shells can hold different maximum numbers of electrons. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electron shell,. What Is The Number Electron Shells.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron What Is The Number Electron Shells The first shell can carry up to two electrons, the second shell can carry up to eight electrons. In the diagram above the energy levels are depicted as the rings around the. Electrons in atoms occupy energy levels, also called electron shells, outside the. Electrons in the same shell have the same amount of energy. The general rule stating the. What Is The Number Electron Shells.
From pressbooks.bccampus.ca
1.5 Electronic Structure of Atoms (Electron Configurations) What Is The Number Electron Shells The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Different shells can hold different maximum numbers of electrons. Electrons in the same shell have the same amount of. What Is The Number Electron Shells.
From brainly.in
draw a diagram of bohrs model of an atom showing four energy shells What Is The Number Electron Shells The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each allowed electron orbit is assigned. Electrons in the same shell have the same amount of energy. The rule to calculate the number of electrons that each shell can hold is 2n 2. The general rule stating the number of electrons present. What Is The Number Electron Shells.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? What Is The Number Electron Shells Electron shells and the bohr model figure 1. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons in the same shell have the same amount of energy. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different maximum numbers of electrons. The first shell is. What Is The Number Electron Shells.
From www.animalia-life.club
Electron Shell Diagram What Is The Number Electron Shells The general rule stating the number of electrons present in a shell is 2n 2,. In the diagram above the energy levels are depicted as the rings around the. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. It should be stressed that there. What Is The Number Electron Shells.
From www.youtube.com
Shells, Subshells, and Orbitals l Understand the difference YouTube What Is The Number Electron Shells The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The general rule stating the number of electrons present in a shell is 2n 2,. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electrons in atoms occupy. What Is The Number Electron Shells.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? What Is The Number Electron Shells It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Different shells can hold different maximum numbers of electrons. Electron shells and the bohr model figure 1. The first. What Is The Number Electron Shells.
From www.slideserve.com
PPT Electron Orbitals PowerPoint Presentation, free download ID650435 What Is The Number Electron Shells The first shell is 2 (1) 2 which gives you 2 electrons. In the diagram above the energy levels are depicted as the rings around the. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. Each allowed electron orbit. What Is The Number Electron Shells.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is The Number Electron Shells Electron shells and the bohr model figure 1. The rule to calculate the number of electrons that each shell can hold is 2n 2. In the diagram above the energy levels are depicted as the rings around the. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Electrons in the same. What Is The Number Electron Shells.
From www.nagwa.com
Question Video Determining the Period Number for Atoms When Given the What Is The Number Electron Shells It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons in the same shell have the same amount of energy. Electrons in atoms occupy energy levels, also called electron shells, outside the. The third. What Is The Number Electron Shells.
From utedzz.blogspot.com
Periodic Table Electrons Shells Periodic Table Timeline What Is The Number Electron Shells It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electron shells and the bohr model figure 1. The general rule stating the number of electrons present in a shell is 2n 2,. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. In. What Is The Number Electron Shells.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Number Electron Shells Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Electrons in the same shell have the same amount of energy. The. What Is The Number Electron Shells.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5413898 What Is The Number Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. Each allowed electron orbit is assigned. In the diagram above the energy levels are depicted as the rings around the. The first shell is 2 (1) 2 which gives you 2 electrons. The general rule stating the number of electrons present in a shell is 2n 2,. The. What Is The Number Electron Shells.
From www.nagwa.com
Question Video Identifying the Maximum Number of Electrons in the 1st What Is The Number Electron Shells Different shells can hold different maximum numbers of electrons. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons in the same shell have the same amount of energy. Electron shells and the bohr model figure 1. Each allowed electron orbit is assigned. Electron shell, regions surrounding the atomic nucleus containing a specific. What Is The Number Electron Shells.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Number Electron Shells The first shell is 2 (1) 2 which gives you 2 electrons. Electrons in the same shell have the same amount of energy. Each allowed electron orbit is assigned. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. Electron. What Is The Number Electron Shells.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Number Electron Shells Electrons in the same shell have the same amount of energy. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. The first shell is 2 (1) 2 which gives you 2 electrons. The rule to calculate the number of electrons that each shell can hold is 2n 2. Different shells can. What Is The Number Electron Shells.
From chemistryatomrevision.weebly.com
Electron Configuration Atomic Structure What Is The Number Electron Shells The general rule stating the number of electrons present in a shell is 2n 2,. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Each allowed electron orbit is assigned. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The first shell. What Is The Number Electron Shells.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID2664384 What Is The Number Electron Shells The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each allowed electron orbit is assigned. Electrons in the same shell have the same amount of energy. Electron shells and the bohr model figure 1. In the diagram above the energy levels are depicted as the rings around the. The first shell. What Is The Number Electron Shells.
From gzscienceclassonline.weebly.com
1. Electron Configuration What Is The Number Electron Shells Electrons in the same shell have the same amount of energy. Each allowed electron orbit is assigned. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The general rule stating the number of electrons present in a shell. What Is The Number Electron Shells.
From www.thoughtco.com
Electron Configuration Chart What Is The Number Electron Shells Electron shells and the bohr model figure 1. Electrons in the same shell have the same amount of energy. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. The first shell can carry up to two electrons, the second. What Is The Number Electron Shells.
From ar.inspiredpencil.com
Number Of Electrons In Each Shell What Is The Number Electron Shells In the diagram above the energy levels are depicted as the rings around the. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. Electrons in the same. What Is The Number Electron Shells.
From brainly.com
what is the electrons, valence electrons, and electron shells number What Is The Number Electron Shells The general rule stating the number of electrons present in a shell is 2n 2,. The first shell is 2 (1) 2 which gives you 2 electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electron shells. What Is The Number Electron Shells.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is The Number Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. The general rule stating the number of electrons present in a shell is 2n 2,. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. The third shell can carry up 18 electrons, but it. What Is The Number Electron Shells.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is The Number Electron Shells The rule to calculate the number of electrons that each shell can hold is 2n 2. Each allowed electron orbit is assigned. Electrons in the same shell have the same amount of energy. Electron shells and the bohr model figure 1. In the diagram above the energy levels are depicted as the rings around the. The third shell can carry. What Is The Number Electron Shells.
From loevwhbck.blob.core.windows.net
What Is Electrons And Shell at Jeff Rios blog What Is The Number Electron Shells The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons in the same shell have the same amount of energy. It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Each allowed electron orbit is assigned. Electron shells and the. What Is The Number Electron Shells.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Number Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Each allowed electron orbit is assigned. Electrons in the same shell have the same amount of energy.. What Is The Number Electron Shells.
From www.allaboutcircuits.com
Quantum Physics Solidstate Device Theory Electronics Textbook What Is The Number Electron Shells It should be stressed that there is a connection between the number of protons in an element, the atomic number that distinguishes one. Different shells can hold different maximum numbers of electrons. Electrons in the same shell have the same amount of energy. The general rule stating the number of electrons present in a shell is 2n 2,. Electron shells. What Is The Number Electron Shells.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table What Is The Number Electron Shells Electron shells and the bohr model figure 1. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different maximum numbers of electrons. Each allowed electron orbit is assigned. The rule to calculate the number of. What Is The Number Electron Shells.
From www.thesciencehive.co.uk
Electron Structure* — the science sauce What Is The Number Electron Shells The first shell is 2 (1) 2 which gives you 2 electrons. In the diagram above the energy levels are depicted as the rings around the. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. The general rule stating the number of electrons present in a shell is 2n 2,. It. What Is The Number Electron Shells.
From chemistrytalk.org
Electron Shells ChemTalk What Is The Number Electron Shells The general rule stating the number of electrons present in a shell is 2n 2,. The first shell is 2 (1) 2 which gives you 2 electrons. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electrons in the same shell have the same amount of energy. Electrons in atoms occupy energy levels, also called electron. What Is The Number Electron Shells.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is The Number Electron Shells The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. Different shells can hold different maximum numbers of electrons. Electrons in atoms occupy energy levels, also called electron shells, outside the. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Each allowed electron. What Is The Number Electron Shells.
From middleschoolscience.com
Patterns of the Periodic Table Finding Shells and Valence Electrons What Is The Number Electron Shells In the diagram above the energy levels are depicted as the rings around the. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electron shells and the bohr model figure 1. The general rule stating the number of electrons present in a shell is 2n 2,. Electrons in atoms occupy energy. What Is The Number Electron Shells.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Number Electron Shells Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different maximum numbers of electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. Electron shell, regions surrounding the atomic nucleus containing a specific number of electrons. Electron shells and the bohr model figure 1.. What Is The Number Electron Shells.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor What Is The Number Electron Shells Electron shells and the bohr model figure 1. Electrons in atoms occupy energy levels, also called electron shells, outside the. The third shell can carry up 18 electrons, but it is more stable by carrying only eight electrons. The first shell can carry up to two electrons, the second shell can carry up to eight electrons. It should be stressed. What Is The Number Electron Shells.