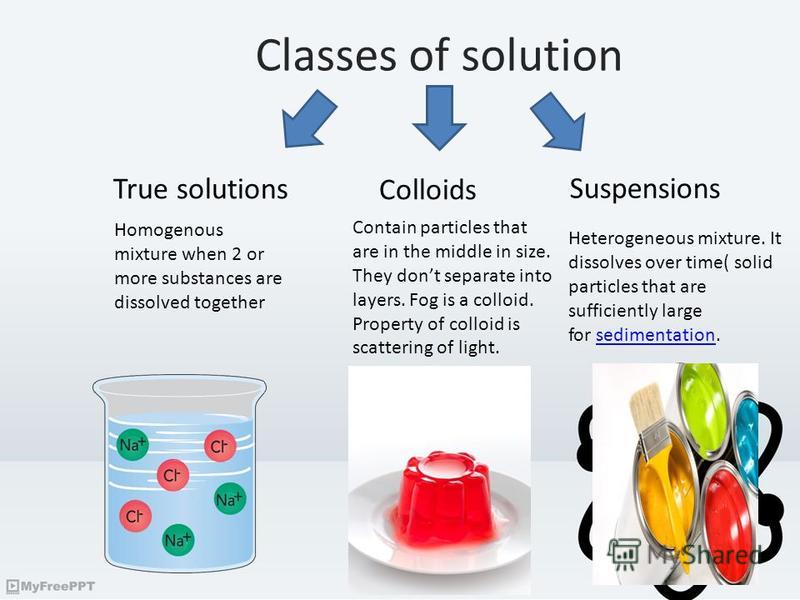
Suspension Of Colloid Mixture . A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is a heterogeneous mixture that settles out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Everyday examples of suspensions include mud, flour in water, and dust in air. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is defined as a homogenous. The dispersed particles separate from the dispersing phase on standing. Colloid particles are not visible and they don’t settle out.
from www.myshared.ru
A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is defined as a homogenous. The dispersed particles separate from the dispersing phase on standing. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in water, and dust in air. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Colloid particles are not visible and they don’t settle out.
Презентация на тему "Colloidal systems. Classes of solution True
Suspension Of Colloid Mixture Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture that settles out upon standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is defined as a homogenous. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles separate from the dispersing phase on standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Everyday examples of suspensions include mud, flour in water, and dust in air. Colloid particles are not visible and they don’t settle out. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a.
From slideplayer.com
BELL WORK! Identify the following as an element, compound, or mixture Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is. Suspension Of Colloid Mixture.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in water, and dust in air. The dispersed particles separate from the dispersing phase on standing. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. These suspensions are heterogeneous. Suspension Of Colloid Mixture.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out upon standing. The dispersed particles separate from the dispersing phase on standing. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. These. Suspension Of Colloid Mixture.
From www.slideserve.com
PPT Mixtures PowerPoint Presentation, free download ID3086482 Suspension Of Colloid Mixture A suspension is defined as a homogenous. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is a heterogeneous mixture that settles out upon standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a. Suspension Of Colloid Mixture.
From www.youtube.com
Solution, Suspension, ColloidTypes of mixtures Chapter 2 Class 9 Suspension Of Colloid Mixture Everyday examples of suspensions include mud, flour in water, and dust in air. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is. Suspension Of Colloid Mixture.
From www.youtube.com
SOLUTION, SUSPENSION AND COLLOID MIXTURES CHEMISTRY 720p YouTube Suspension Of Colloid Mixture These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. The dispersed particles separate from the dispersing phase on standing. Both suspensions and colloids are heterogeneous mixtures,. Suspension Of Colloid Mixture.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Of Colloid Mixture Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The dispersed particles separate from the dispersing phase on standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in. Suspension Of Colloid Mixture.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. These suspensions are heterogeneous mixtures composed of relatively. Suspension Of Colloid Mixture.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Of Colloid Mixture Colloid particles are not visible and they don’t settle out. Everyday examples of suspensions include mud, flour in water, and dust in air. The dispersed particles separate from the dispersing phase on standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is a heterogeneous mixture of particles. Suspension Of Colloid Mixture.
From philschatz.com
Colloids · Chemistry Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Colloid particles are not visible and they don’t settle out. A suspension is defined as a homogenous. Everyday examples of suspensions include mud, flour in water, and dust in air. A suspension is a heterogeneous mixture that settles. Suspension Of Colloid Mixture.
From ar.inspiredpencil.com
Colloids Suspensions And Solutions Suspension Of Colloid Mixture The dispersed particles separate from the dispersing phase on standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are not. Suspension Of Colloid Mixture.
From ar.inspiredpencil.com
Suspension Mixture Suspension Of Colloid Mixture The dispersed particles separate from the dispersing phase on standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A suspension is a heterogeneous mixture that settles out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is defined. Suspension Of Colloid Mixture.
From www.vecteezy.com
Coagulation of Colloid Particles vector illustration 21669350 Vector Suspension Of Colloid Mixture Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A suspension is a heterogeneous mixture that settles out upon standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. A suspension is defined as a heterogeneous mixture in. Suspension Of Colloid Mixture.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with. Suspension Of Colloid Mixture.
From www.pinterest.com
mixture suspension colloid Google Search Chemistry worksheets Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. A suspension is defined as a homogenous. The dispersed particles separate from the dispersing phase on standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Here is how to distinguish among solutions, suspensions, colloids, and other. Suspension Of Colloid Mixture.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are not visible and they don’t settle out. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Everyday. Suspension Of Colloid Mixture.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Suspension Of Colloid Mixture Colloid particles are not visible and they don’t settle out. A suspension is defined as a homogenous. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The dispersed particles separate from the dispersing phase. Suspension Of Colloid Mixture.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Of Colloid Mixture A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. The dispersed particles separate from the dispersing phase on standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Here is how to distinguish among solutions, suspensions, colloids, and other dispersions. Suspension Of Colloid Mixture.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Of Colloid Mixture Colloid particles are not visible and they don’t settle out. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is defined as a homogenous. A suspension is a heterogeneous mixture that settles out upon standing. Here is how to distinguish among solutions, suspensions, colloids, and. Suspension Of Colloid Mixture.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Of Colloid Mixture A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in water, and dust in air. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension. Suspension Of Colloid Mixture.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Of Colloid Mixture A suspension is defined as a homogenous. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Colloid particles are not visible and they don’t settle out. These suspensions are heterogeneous mixtures. Suspension Of Colloid Mixture.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. Everyday examples of suspensions include mud, flour in water, and dust in air. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second. Suspension Of Colloid Mixture.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Of Colloid Mixture Colloid particles are not visible and they don’t settle out. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture that settles out upon standing. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. The. Suspension Of Colloid Mixture.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Of Colloid Mixture Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles separate from the dispersing phase on standing. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is a heterogeneous mixture that settles out. Suspension Of Colloid Mixture.
From www.numerade.com
SOLVED The images depict solid particles of different diameters in a Suspension Of Colloid Mixture Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles. Suspension Of Colloid Mixture.
From www.pinterest.com
Colloid Easy Science Physics concepts, Organic chemistry study Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is defined as. Suspension Of Colloid Mixture.
From slideplayer.com
Covalent Bonds Covalent bond ppt download Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. The dispersed particles separate from the dispersing phase on standing. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid. Suspension Of Colloid Mixture.
From www.slideshare.net
Mixtures (2) Suspension Of Colloid Mixture A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. Colloid particles are not visible and they don’t settle out. A suspension is defined as a homogenous. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying.. Suspension Of Colloid Mixture.
From suspensioncolloid-00.blogspot.com
SuspensionColloid Suspension Of Colloid Mixture Everyday examples of suspensions include mud, flour in water, and dust in air. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. A suspension is defined. Suspension Of Colloid Mixture.
From www.slideserve.com
PPT SOLUTION CHEMISTRY PowerPoint Presentation, free download ID Suspension Of Colloid Mixture A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. The dispersed particles separate from the dispersing phase on standing. A suspension is a heterogeneous mixture that settles out upon standing. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those. Suspension Of Colloid Mixture.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension Of Colloid Mixture Colloid particles are not visible and they don’t settle out. A suspension is defined as a homogenous. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture that settles. Suspension Of Colloid Mixture.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Suspension Of Colloid Mixture A suspension is a heterogeneous mixture that settles out upon standing. Colloid particles are not visible and they don’t settle out. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; These suspensions are heterogeneous mixtures composed of relatively large particles that are visible (or that can be seen with a magnifying. Here is. Suspension Of Colloid Mixture.
From www.pinterest.com
solutionssuspensionsandcolloids9728.jpg (728×546) Science Suspension Of Colloid Mixture A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. Here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. The dispersed particles separate from the dispersing phase on standing. Everyday examples of suspensions include mud, flour in water,. Suspension Of Colloid Mixture.
From www.youtube.com
HETEROGENEOUS mixture l suspension l colloid l immersion l MELC l S6MT Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. A suspension is defined as a heterogeneous mixture in which the solid particles are spread throughout the liquid without dissolving in it. A suspension is a heterogeneous mixture that settles out upon standing. The dispersed particles separate from. Suspension Of Colloid Mixture.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Suspension Of Colloid Mixture A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Everyday examples of suspensions include mud, flour in water, and dust in air. A suspension is a heterogeneous mixture that settles out upon standing. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension. Suspension Of Colloid Mixture.