Lab Ideal Gas Law Answers . the ideal gas law. The temperature of the water. 20.0g/100ml x 35.0ml = 7.g. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. an ideal gas follows the ideal gas law at all conditions of p and t. the purpose of the experiment is to experimentally determine the ideal gas constant r. Which gas law is this. The ideal gas law relates all the independent properties of a gas under any particular condition. The particles in an ideal gas do not have finite. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. Use the ideal gas constant to.
from www.slideserve.com
Which gas law is this. The particles in an ideal gas do not have finite. calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. 20.0g/100ml x 35.0ml = 7.g. the ideal gas law. an ideal gas follows the ideal gas law at all conditions of p and t. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). The temperature of the water. Use the ideal gas constant to. The ideal gas law relates all the independent properties of a gas under any particular condition.
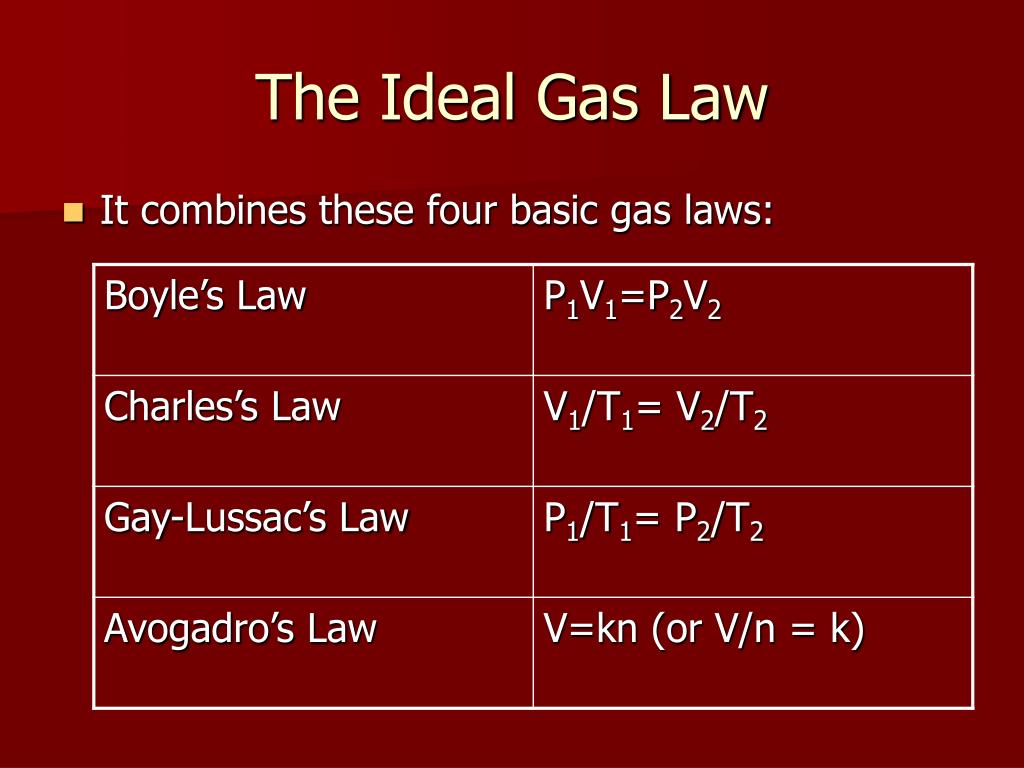
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594
Lab Ideal Gas Law Answers an ideal gas follows the ideal gas law at all conditions of p and t. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The particles in an ideal gas do not have finite. The temperature of the water. The ideal gas law relates all the independent properties of a gas under any particular condition. Use the ideal gas constant to. 20.0g/100ml x 35.0ml = 7.g. Which gas law is this. the ideal gas law. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). the purpose of the experiment is to experimentally determine the ideal gas constant r. an ideal gas follows the ideal gas law at all conditions of p and t.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe Lab Ideal Gas Law Answers gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. 20.0g/100ml x 35.0ml = 7.g. the purpose of the experiment is to experimentally determine the ideal gas constant r. many real gases behave like an ideal gas under various conditions (for example. Lab Ideal Gas Law Answers.
From crosweaphari.weebly.com
Ideal Gas Law Chem Worksheet 144 Answer Key LINK Lab Ideal Gas Law Answers an ideal gas follows the ideal gas law at all conditions of p and t. the purpose of the experiment is to experimentally determine the ideal gas constant r. Which gas law is this. Use the ideal gas constant to. The particles in an ideal gas do not have finite. 20.0g/100ml x 35.0ml = 7.g. calculate the. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved CHEMISTRY. IDEAL GAS LAW CONSTANT INTRODUCTION Lab Ideal Gas Law Answers gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. an ideal gas follows the ideal gas law at all conditions of p and t. the purpose of the experiment is to experimentally determine the ideal gas constant r. The temperature of. Lab Ideal Gas Law Answers.
From answerfullcookout.z13.web.core.windows.net
Ideal Gas Law Lab Answer Key Lab Ideal Gas Law Answers the ideal gas law. The particles in an ideal gas do not have finite. The ideal gas law relates all the independent properties of a gas under any particular condition. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). calculate the mass of nacl needed to prep 35.0. Lab Ideal Gas Law Answers.
From learningschoolgraciauwb.z4.web.core.windows.net
Ideal Gas Law Practice Questions Lab Ideal Gas Law Answers The temperature of the water. an ideal gas follows the ideal gas law at all conditions of p and t. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are. Lab Ideal Gas Law Answers.
From myans.bhantedhammika.net
Ideal Gas Laws Worksheet Answer Key Lab Ideal Gas Law Answers Which gas law is this. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. Use the ideal gas constant to. The ideal gas law relates all the independent properties of a gas under any particular condition. in the ideal gas law lab,. Lab Ideal Gas Law Answers.
From www.studyxapp.com
chemistry ideal gas law constant introduction laboratory simulation a Lab Ideal Gas Law Answers Which gas law is this. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The ideal gas law relates all the independent properties of a gas under any particular condition. The particles in an ideal gas do not have finite. an ideal. Lab Ideal Gas Law Answers.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Lab Ideal Gas Law Answers Which gas law is this. The ideal gas law relates all the independent properties of a gas under any particular condition. an ideal gas follows the ideal gas law at all conditions of p and t. Use the ideal gas constant to. the purpose of the experiment is to experimentally determine the ideal gas constant r. The particles. Lab Ideal Gas Law Answers.
From www.youtube.com
Ideal Gas Law Example Question YouTube Lab Ideal Gas Law Answers calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. the purpose of the experiment is to experimentally determine the ideal gas constant r. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? an ideal gas follows the ideal gas law at all conditions of. Lab Ideal Gas Law Answers.
From www.pinterest.com
Pin by Jessica Joyce on Ideal Gas Law Ideal gas law, Gas laws Lab Ideal Gas Law Answers The ideal gas law relates all the independent properties of a gas under any particular condition. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The temperature of the water. 20.0g/100ml x 35.0ml = 7.g. an ideal gas follows the ideal gas. Lab Ideal Gas Law Answers.
From sciencenotes.org
Ideal Gas Law Formula and Examples Lab Ideal Gas Law Answers an ideal gas follows the ideal gas law at all conditions of p and t. the ideal gas law. Use the ideal gas constant to. The particles in an ideal gas do not have finite. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? 20.0g/100ml x 35.0ml = 7.g. the. Lab Ideal Gas Law Answers.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Lab Ideal Gas Law Answers Which gas law is this. calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. the ideal gas law. Use the ideal gas constant to. The particles in an ideal gas do not have finite. The ideal gas law relates all the independent properties of a gas under any particular condition. The temperature. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved GAS LAW • IDEAL GAS LAW CONSTANT INTRODUCTION Lab Ideal Gas Law Answers Which gas law is this. The temperature of the water. The ideal gas law relates all the independent properties of a gas under any particular condition. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? many real gases behave like an ideal gas under various conditions (for example high temperature or low. Lab Ideal Gas Law Answers.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Lab Ideal Gas Law Answers in the ideal gas law lab, how is the temperature of the hydrogen gas determined? an ideal gas follows the ideal gas law at all conditions of p and t. the purpose of the experiment is to experimentally determine the ideal gas constant r. The particles in an ideal gas do not have finite. 20.0g/100ml x 35.0ml. Lab Ideal Gas Law Answers.
From www.pinterest.com
More Gas Laws Worksheet Charles Law Worksheet Answer Key Chemistry Lab Ideal Gas Law Answers the purpose of the experiment is to experimentally determine the ideal gas constant r. The temperature of the water. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. the ideal gas law. The ideal gas law relates all the independent properties. Lab Ideal Gas Law Answers.
From studylib.net
Lab 6 The Ideal Gas Law Lab Ideal Gas Law Answers many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The particles in an ideal gas do not have finite. The ideal gas law relates. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved Experiment 10 The Ideal Gas Law Constant (R) PostLab Lab Ideal Gas Law Answers Use the ideal gas constant to. 20.0g/100ml x 35.0ml = 7.g. Which gas law is this. the ideal gas law. calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. The ideal gas law relates all the independent properties of a gas under any particular condition. gases whose properties of p, v,. Lab Ideal Gas Law Answers.
From www.studypool.com
SOLUTION Ideal Gas Lab Report Studypool Lab Ideal Gas Law Answers calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. an ideal gas follows the ideal gas law at all conditions of p and t. the ideal gas law. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? The particles in an ideal gas do. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved Part I Using the Ideal Gas Law Experiment 1 Lab Ideal Gas Law Answers calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. The particles in an ideal gas do not have finite. Which gas law is this. The temperature of the water. an ideal gas follows the ideal gas law at all conditions of p and t. in the ideal gas law lab, how. Lab Ideal Gas Law Answers.
From letra759.blogspot.com
[28+] Phet Gas Law Simulation Lab Answer Key, Gas Laws PHET Simulation Lab Ideal Gas Law Answers Use the ideal gas constant to. The ideal gas law relates all the independent properties of a gas under any particular condition. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). The temperature of the water. calculate the mass of nacl needed to prep 35.0 ml of a 20.0%. Lab Ideal Gas Law Answers.
From studylib.net
Gas Laws Worksheet Answer Key Lab Ideal Gas Law Answers the ideal gas law. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? The particles in an ideal gas do not have finite. The ideal gas law relates all the independent properties of a gas under any particular condition. Which gas law is this. many real gases behave like an ideal. Lab Ideal Gas Law Answers.
From askworksheet.com
Ideal Gas Law Worksheet Answer Key Askworksheet Lab Ideal Gas Law Answers Use the ideal gas constant to. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? The temperature of the water. an ideal gas follows the ideal gas law at all conditions of p and t. The particles in an ideal gas do not have finite. calculate the mass of nacl needed. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved Gas Laws and Stoichiometry Prelab Prelab Calculations Lab Ideal Gas Law Answers many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). in the ideal gas law lab, how is the temperature of the hydrogen gas determined? the purpose of the experiment is to experimentally determine the ideal gas constant r. The ideal gas law relates all the independent properties of. Lab Ideal Gas Law Answers.
From www.vrogue.co
Solution Ideal Gas Law Lab Studypool vrogue.co Lab Ideal Gas Law Answers in the ideal gas law lab, how is the temperature of the hydrogen gas determined? The temperature of the water. The ideal gas law relates all the independent properties of a gas under any particular condition. 20.0g/100ml x 35.0ml = 7.g. an ideal gas follows the ideal gas law at all conditions of p and t. many. Lab Ideal Gas Law Answers.
From lessoncampusencodes.z21.web.core.windows.net
Ideal Gas Law Stoichiometry Worksheet Lab Ideal Gas Law Answers many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? Which gas law is this. The temperature of the. Lab Ideal Gas Law Answers.
From worksheets.decoomo.com
20++ Ideal Gas Law Worksheet Answers Worksheets Decoomo Lab Ideal Gas Law Answers The ideal gas law relates all the independent properties of a gas under any particular condition. 20.0g/100ml x 35.0ml = 7.g. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? Use the ideal gas constant to. an ideal gas follows the ideal gas law at all conditions of p and t. . Lab Ideal Gas Law Answers.
From www.worksheeto.com
13 Best Images of Pressure Problems Worksheet Answer Key Lab Ideal Gas Law Answers gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. Use the ideal gas constant to. in the ideal gas law lab, how is the temperature of the hydrogen gas determined? many real gases behave like an ideal gas under various conditions. Lab Ideal Gas Law Answers.
From chem.libretexts.org
Chapter 10.6 Stoichiometry Involving Gases Chemistry LibreTexts Lab Ideal Gas Law Answers gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. 20.0g/100ml x 35.0ml = 7.g. the purpose of the experiment is to experimentally determine the ideal gas constant r. Which gas law is this. the ideal gas law. The temperature of the. Lab Ideal Gas Law Answers.
From www.studypool.com
SOLUTION Ideal Gas Law Multiple Choice Questions Studypool Lab Ideal Gas Law Answers The particles in an ideal gas do not have finite. the ideal gas law. The ideal gas law relates all the independent properties of a gas under any particular condition. an ideal gas follows the ideal gas law at all conditions of p and t. many real gases behave like an ideal gas under various conditions (for. Lab Ideal Gas Law Answers.
From studyschoolford.z21.web.core.windows.net
Ideal Gas Law Notes Lab Ideal Gas Law Answers an ideal gas follows the ideal gas law at all conditions of p and t. the purpose of the experiment is to experimentally determine the ideal gas constant r. the ideal gas law. The temperature of the water. 20.0g/100ml x 35.0ml = 7.g. calculate the mass of nacl needed to prep 35.0 ml of a 20.0%. Lab Ideal Gas Law Answers.
From www.chegg.com
CHEM10 Lab 8 Using the Ideal Gas Law Table 2 Lab Ideal Gas Law Answers in the ideal gas law lab, how is the temperature of the hydrogen gas determined? Use the ideal gas constant to. The temperature of the water. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The particles in an ideal gas do. Lab Ideal Gas Law Answers.
From www.studypool.com
SOLUTION Ideal Gas Law Lab Studypool Lab Ideal Gas Law Answers 20.0g/100ml x 35.0ml = 7.g. an ideal gas follows the ideal gas law at all conditions of p and t. The ideal gas law relates all the independent properties of a gas under any particular condition. Which gas law is this. Use the ideal gas constant to. calculate the mass of nacl needed to prep 35.0 ml of. Lab Ideal Gas Law Answers.
From studylib.net
ideal gas law lab Lab Ideal Gas Law Answers an ideal gas follows the ideal gas law at all conditions of p and t. the ideal gas law. gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. calculate the mass of nacl needed to prep 35.0 ml of a. Lab Ideal Gas Law Answers.
From www.chegg.com
Solved CHEMISTRY IDEAL GAS LAW CONSTANT SUBMIT Lab Ideal Gas Law Answers gases whose properties of p, v, and t are accurately described by the ideal gas law (or the other gas laws) are said to exhibit. The particles in an ideal gas do not have finite. the ideal gas law. 20.0g/100ml x 35.0ml = 7.g. The ideal gas law relates all the independent properties of a gas under any. Lab Ideal Gas Law Answers.
From www.studocu.com
Ideal Gas Law Labster Lab OL Lab 7 Ideal Gas Law Learning Lab Ideal Gas Law Answers The ideal gas law relates all the independent properties of a gas under any particular condition. calculate the mass of nacl needed to prep 35.0 ml of a 20.0% nacl solution. Use the ideal gas constant to. many real gases behave like an ideal gas under various conditions (for example high temperature or low pressure). 20.0g/100ml x 35.0ml. Lab Ideal Gas Law Answers.