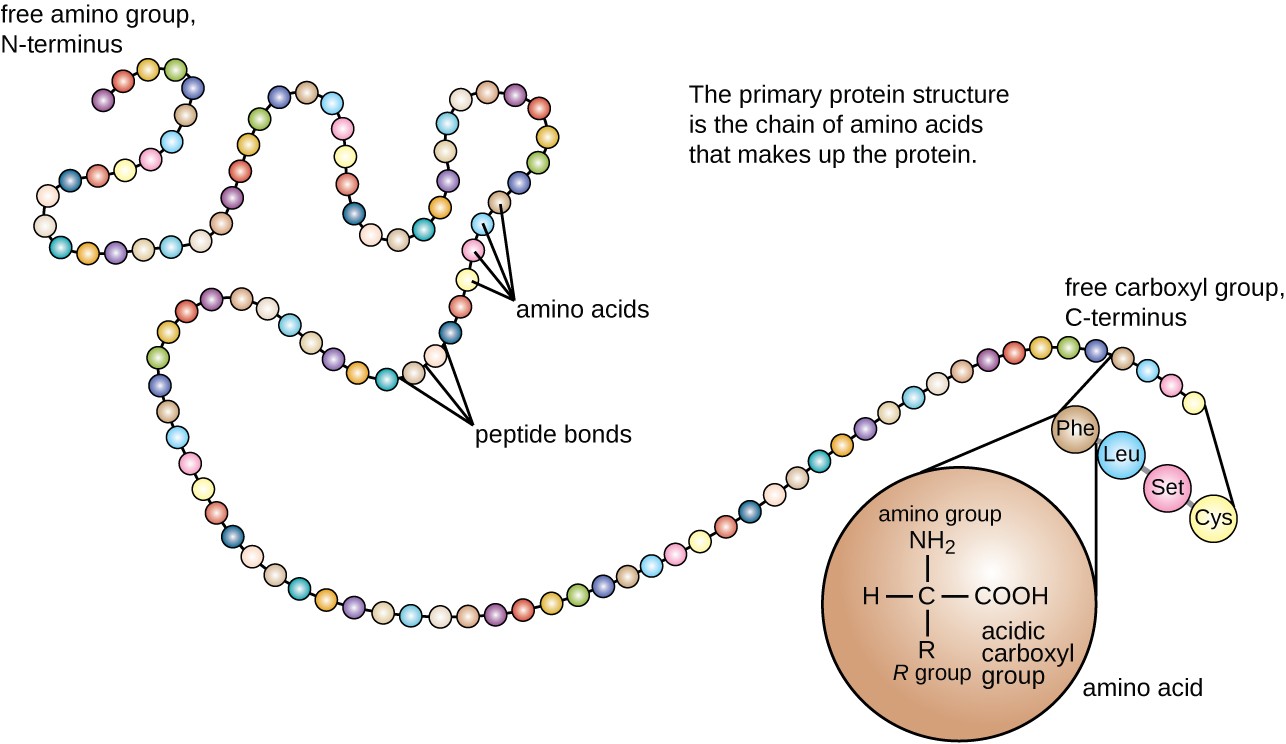
Protein Folding Organelle . Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles.
from courses.lumenlearning.com
Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the er. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from.
Proteins Microbiology
Protein Folding Organelle Protein folding is optimized in the er. Protein folding is optimized in the er. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a.
From www.youtube.com
Protein folding explained YouTube Protein Folding Organelle Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. To ensure protein folding fidelity. Protein Folding Organelle.
From www.researchgate.net
Protein secretion Ribosomes deposits the protein in endoplasmic Protein Folding Organelle To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Members of the hsp70 and hsp60 families. Protein Folding Organelle.
From www.britannica.com
6 Cell Organelles Britannica Protein Folding Organelle To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as. Protein Folding Organelle.
From study.com
Organelles Involved in Protein Synthesis Video & Lesson Transcript Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the. Protein Folding Organelle.
From courses.lumenlearning.com
Proteins Microbiology Protein Folding Organelle Protein folding is optimized in the er. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones. Protein Folding Organelle.
From www.slideserve.com
PPT Chapter 5 Introduction to Nanobiology PowerPoint Presentation Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent work has provided fascinating. Protein Folding Organelle.
From jonlieffmd.com
Protein Folding in the Neuron and the Mind Jon Lieff, M.D. Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as. Protein Folding Organelle.
From www.pinterest.com
Protein Import into ER in 2020 Cell biology, Mcat study, Anatomy and Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Proteins such. Protein Folding Organelle.
From en.wikipedia.org
Protein biosynthesis Wikipedia Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to. Protein Folding Organelle.
From designarchitects.art
Introduction To Protein Architecture The Structural Biology Of Proteins Protein Folding Organelle Protein folding is optimized in the er. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes. Protein Folding Organelle.
From www.pinterest.com
Endo reticulum Structure and function, Cell structure, Physiology Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Protein folding is optimized in the er. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones. Protein Folding Organelle.
From www.expii.com
Amino Acids & Polypeptide Chains — Structure & Synthesis Expii Protein Folding Organelle Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Protein folding is optimized in the er. Recent. Protein Folding Organelle.
From courses.lumenlearning.com
Eukaryotic Cells Biology I Protein Folding Organelle Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Proteins such. Protein Folding Organelle.
From www.science.org
How FastFolding Proteins Fold Science Protein Folding Organelle To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Protein folding is optimized in the er. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin. Protein Folding Organelle.
From www.slideserve.com
PPT Protein Sorting PowerPoint Presentation, free download ID847737 Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to. Protein Folding Organelle.
From discover.hubpages.com
The Cell Theory & Structure HubPages Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric. Protein Folding Organelle.
From owlcation.com
Structure of a Plant Cell A Visual Guide Owlcation Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent work has provided fascinating insight into the process of protein folding. Protein Folding Organelle.
From www.ebi.ac.uk
Fold Biomacromolecular structures Protein Folding Organelle Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures. Protein Folding Organelle.
From www.frontiersin.org
Frontiers Protein folding as a driving force for dual protein Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the er. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to. Protein Folding Organelle.
From www.slideserve.com
PPT Protein Folding and Processing PowerPoint Presentation, free Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the er. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric. Protein Folding Organelle.
From www.researchgate.net
Protein folding inside the cell a new protein is synthetized at the Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the. Protein Folding Organelle.
From gamma.app
Protein Folding Gamma Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Protein folding is optimized in the er. To ensure protein folding fidelity. Protein Folding Organelle.
From www.britannica.com
Cell Endoplasmic Reticulum, Organelles, Membranes Britannica Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Protein folding is optimized in the er. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from.. Protein Folding Organelle.
From mavink.com
Protein Folding Pathway Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to. Protein Folding Organelle.
From www.slideserve.com
PPT Protein Folding and Processing PowerPoint Presentation, free Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. The. Protein Folding Organelle.
From www.genesispark.com
Protein Machines Part 3 Genesis Park Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. The. Protein Folding Organelle.
From www.researchgate.net
Protein folding from primary to tertiary structure [12]. Download Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. The accumulation. Protein Folding Organelle.
From www.slideserve.com
PPT Molecular Biology Primer PowerPoint Presentation, free download Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding. Protein Folding Organelle.
From courses.lumenlearning.com
Proteins Microbiology Protein Folding Organelle The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the er. Recent work has provided fascinating. Protein Folding Organelle.
From www.slideserve.com
PPT Protein Sorting PowerPoint Presentation, free download ID847737 Protein Folding Organelle Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells. Protein Folding Organelle.
From knowt.com
Proteins Notes Knowt Protein Folding Organelle To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Protein folding is optimized in the. Protein Folding Organelle.
From www.haikudeck.com
Folding Proteins by Brittany Goodeaux Protein Folding Organelle Protein folding is optimized in the er. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes. Protein Folding Organelle.
From www.lecturio.com
The Cell Organelles Concise Medical Knowledge Protein Folding Organelle Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures from. Recent work has provided fascinating insight into the process of protein folding on the ribosome and revealed how highly allosteric chaperones such as the heat shock protein 70 (hsp70), hsp90, and chaperonin systems modulate the folding energy landscapes of their protein clients. The. Protein Folding Organelle.
From rsscience.com
Cell Organelles and their Functions Rs' Science Protein Folding Organelle Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. Protein folding is optimized in the er. Proteins such as calnexin can temporarily bind to nascent polypeptides, preventing them from forming secondary structures. Protein Folding Organelle.
From slideplayer.com
CHAPTER 6 A TOUR OF THE CELL ppt download Protein Folding Organelle To ensure protein folding fidelity and to maintain er functions, the unfolded protein response (upr) of eukaryotic cells evolved to a. The accumulation of unfolded/misfolded proteins in the er activates signaling events to orchestrate adaptive cellular. Protein folding is optimized in the er. Members of the hsp70 and hsp60 families are found in the cytosol and in subcellular organelles. Recent. Protein Folding Organelle.