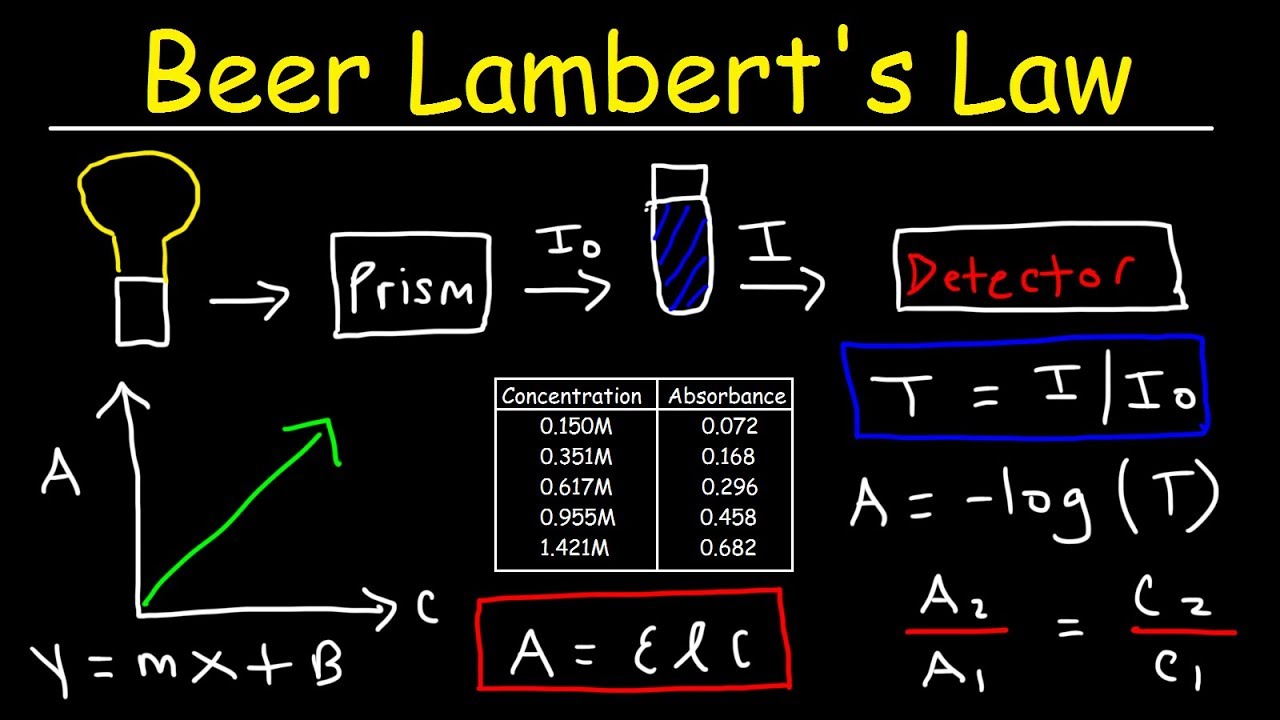
Beer Lambert Law Relationship . The absorbance (a) is a unitless number because io i i o i is unitless. The absorbance depends on the concentration (c c) and the path length (l l). In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\mathrm{a = \varepsilon bc} \nonumber \] The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration.
from www.youtube.com
The absorbance depends on the concentration (c c) and the path length (l l). The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\mathrm{a = \varepsilon bc} \nonumber \] It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The attenuation of light occurs as a result of distance through solution or increasing concentration. The absorbance (a) is a unitless number because io i i o i is unitless.
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry
Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The absorbance depends on the concentration (c c) and the path length (l l). in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. \[\mathrm{a = \varepsilon bc} \nonumber \] The attenuation of light occurs as a result of distance through solution or increasing concentration. The absorbance (a) is a unitless number because io i i o i is unitless. The premise is that a light beam becomes weaker as it passes through a chemical solution.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Relationship in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance (a) is a unitless number because io i i o i is unitless. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The. Beer Lambert Law Relationship.
From dxokacddk.blob.core.windows.net
Beer's Law Absorption at Ellen Day blog Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation. Beer Lambert Law Relationship.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through. Beer Lambert Law Relationship.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Relationship The attenuation of light occurs as a result of distance through solution or increasing concentration. The absorbance (a) is a unitless number because io i i o i is unitless. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. It provides a mathematical. Beer Lambert Law Relationship.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. \[\mathrm{a = \varepsilon bc} \nonumber \] in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The attenuation of light occurs as a result of distance through solution or. Beer Lambert Law Relationship.
From www.youtube.com
Beer Lambert Law, Molar Extinction Coefficient, Spectrophotometry YouTube Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\mathrm{a = \varepsilon bc} \nonumber \] It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The absorbance depends on the concentration (c c) and the path length (l l). In other words, a solution absorbs. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Determining An Equilibrium Constant Using Spectrophotometry and Beer Lambert Law Relationship beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The absorbance (a) is a unitless number because io i i o i is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. The premise is that a light beam. Beer Lambert Law Relationship.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\mathrm{a = \varepsilon bc} \nonumber \] It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The premise is that a light. Beer Lambert Law Relationship.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. The absorbance (a) is a unitless number because io i i o i is unitless. The absorbance depends on the concentration (c c) and the path length (l l). beer's law states that a chemical solution's concentration is directly proportional to its light. Beer Lambert Law Relationship.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer Lambert Law Relationship in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance depends on the concentration. Beer Lambert Law Relationship.
From exolwzeip.blob.core.windows.net
Beer's Law Equation Chemistry at Taylor Jones blog Beer Lambert Law Relationship The attenuation of light occurs as a result of distance through solution or increasing concentration. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The absorbance depends on the concentration (c c) and the path length (l l). beer's law states that a chemical solution's concentration is directly proportional to. Beer Lambert Law Relationship.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The absorbance depends on the concentration (c c) and the path length (l l). It provides a mathematical relationship between the substance’s concentration in a solution and its. Beer Lambert Law Relationship.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law Relationship in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance depends on the concentration (c c) and the path length (l l). The attenuation of light occurs as a result of distance through solution or increasing concentration. It provides a mathematical relationship. Beer Lambert Law Relationship.
From www.youtube.com
Spectrophotometric terms and BeerLambert Law YouTube Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. In other words,. Beer Lambert Law Relationship.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law Relationship beer's law states that a chemical solution's concentration is directly proportional to its light absorption. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\mathrm{a = \varepsilon bc} \nonumber \] The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a. Beer Lambert Law Relationship.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Relationship in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. The absorbance (a) is a unitless number because io i i o i is unitless. \[\mathrm{a = \varepsilon bc} \nonumber \] In other words, a solution absorbs more monochromatic light the further it passes. Beer Lambert Law Relationship.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. The premise is that a light beam becomes weaker as it passes through a chemical solution. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. It provides a. Beer Lambert Law Relationship.
From www.youtube.com
Analytical Instrumentation Tutorial 2 Beer Lambert Law YouTube Beer Lambert Law Relationship The absorbance depends on the concentration (c c) and the path length (l l). \[\mathrm{a = \varepsilon bc} \nonumber \] The premise is that a light beam becomes weaker as it passes through a chemical solution. The absorbance (a) is a unitless number because io i i o i is unitless. It provides a mathematical relationship between the substance’s concentration. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Lab 6 Saliva Practical BeerLambert Law PowerPoint Presentation Beer Lambert Law Relationship The absorbance depends on the concentration (c c) and the path length (l l). The absorbance (a) is a unitless number because io i i o i is unitless. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in spectroscopy, beer’s law states that the absorption of light by a sample is. Beer Lambert Law Relationship.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Relationship In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The attenuation of light occurs as a result of distance through solution or increasing concentration. The absorbance depends on the. Beer Lambert Law Relationship.
From slidetodoc.com
Spectrophotometry Key Concepts Lamberts Law of Absorption Beers Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. It provides a mathematical relationship between. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Relationship The attenuation of light occurs as a result of distance through solution or increasing concentration. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The absorbance depends on the concentration (c c) and the path. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation ID2407461 Beer Lambert Law Relationship The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance depends on the concentration (c c) and the path length (l l). The absorbance (a) is a unitless number because io i i o i is unitless. In other words, a solution absorbs more monochromatic light. Beer Lambert Law Relationship.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\mathrm{a = \varepsilon bc} \nonumber \] The premise is that a light beam becomes weaker as it passes through a chemical solution. It. Beer Lambert Law Relationship.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Relationship It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\mathrm{a = \varepsilon bc} \nonumber \] beer's law states that a chemical solution's concentration is directly proportional to its light absorption. In other words, a solution. Beer Lambert Law Relationship.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation ID2407366 Beer Lambert Law Relationship The absorbance depends on the concentration (c c) and the path length (l l). In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. in spectroscopy, beer’s law states. Beer Lambert Law Relationship.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Relationship \[\mathrm{a = \varepsilon bc} \nonumber \] In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. The premise is that a light beam becomes weaker as it passes through a chemical solution. The absorbance (a) is a unitless number because io i i o i is unitless. It. Beer Lambert Law Relationship.
From studylib.net
Beer Lambert Law Beer Lambert Law Relationship It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance (a) is a unitless number because io i i o i is. Beer Lambert Law Relationship.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer Lambert Law Relationship The absorbance (a) is a unitless number because io i i o i is unitless. The absorbance depends on the concentration (c c) and the path length (l l). It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. In other words, a solution absorbs more monochromatic light the further it passes. Beer Lambert Law Relationship.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Relationship In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance (a) is a unitless number because io i i o i is unitless. beer's. Beer Lambert Law Relationship.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Relationship \[\mathrm{a = \varepsilon bc} \nonumber \] The attenuation of light occurs as a result of distance through solution or increasing concentration. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its concentration. beer's law states that a chemical solution's concentration is directly proportional to. Beer Lambert Law Relationship.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Relationship beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. The absorbance (a) is a unitless number because io i i o i is unitless. in spectroscopy, beer’s law states that the absorption of light by a. Beer Lambert Law Relationship.
From www.youtube.com
Beer Law Lambert Law Limiting Law Absorbance Transmittance Beer Lambert Law Relationship In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance depends on the concentration (c c) and the path length (l l). beer's law states that a chemical solution's concentration is directly proportional to its light absorption. in. Beer Lambert Law Relationship.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Relationship The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. It provides a mathematical relationship between the substance’s concentration in a solution and its ability to absorb light. \[\mathrm{a = \varepsilon bc} \nonumber \] The absorbance depends on the. Beer Lambert Law Relationship.