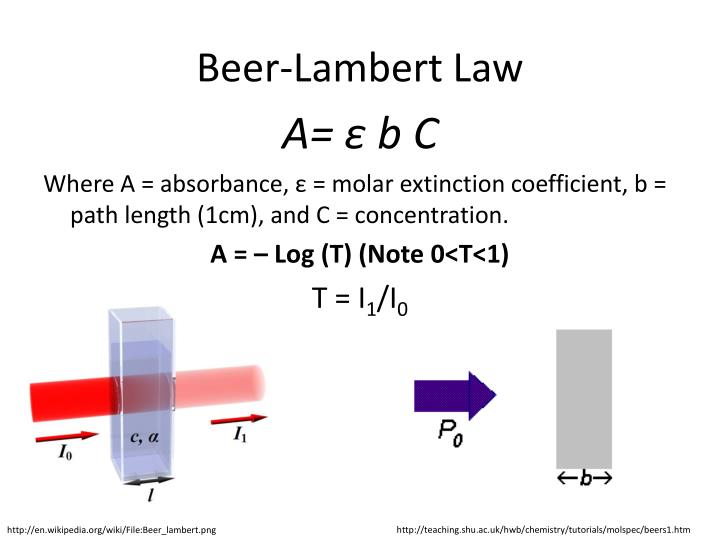
Beer Lambert Law Graph Explained . while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. beer's law is an equation that relates light's attenuation to a material's properties. One of the most basic of these. “the path length and concentration of a chemical are directly proportional to its absorption of light.” in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. beer’s law suggests that a plot of absorbance vs. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The law states that a chemical's concentration is directly proportional to a solution's absorbance.
from www.slideserve.com
in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. One of the most basic of these. The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer's law is an equation that relates light's attenuation to a material's properties. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer’s law suggests that a plot of absorbance vs. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into.
PPT Determining the Concentration of a Solution Beer’s Law
Beer Lambert Law Graph Explained \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. One of the most basic of these. beer's law is an equation that relates light's attenuation to a material's properties. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. beer’s law suggests that a plot of absorbance vs.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Graph Explained in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. One of the most basic of these. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we. Beer Lambert Law Graph Explained.
From dxowmggun.blob.core.windows.net
LambertBeer's Law Was Described To A General Equation at Thomas Partin Beer Lambert Law Graph Explained The law states that a chemical's concentration is directly proportional to a solution's absorbance. “the path length and concentration of a chemical are directly proportional to its absorption of light.” You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. since the concentration, path length and molar absorptivity are all. Beer Lambert Law Graph Explained.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Beer Lambert Law Graph Explained in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. beer's law is an equation that relates light's attenuation to a material's properties. “the path length and concentration of a chemical. Beer Lambert Law Graph Explained.
From calculatorghw.blogspot.com
Beer Lambert Law Calculator CALCULATOR GHW Beer Lambert Law Graph Explained since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer lamberts law states a relationship between the. Beer Lambert Law Graph Explained.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Graph Explained in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. One of the most basic of these. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is. Beer Lambert Law Graph Explained.
From dxopxkghh.blob.core.windows.net
Beer's Law Of Spectrophotometry at Ella Yeh blog Beer Lambert Law Graph Explained since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. The law. Beer Lambert Law Graph Explained.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer Lambert Law Graph Explained while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer's law is an equation that relates light's attenuation to a material's properties. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance. Beer Lambert Law Graph Explained.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Graph Explained One of the most basic of these. “the path length and concentration of a chemical are directly proportional to its absorption of light.” in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations. Beer Lambert Law Graph Explained.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer Lambert Law Graph Explained One of the most basic of these. “the path length and concentration of a chemical are directly proportional to its absorption of light.” You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation,. Beer Lambert Law Graph Explained.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer Lambert Law Graph Explained in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. beer’s law suggests that a plot of absorbance vs. The law states that a chemical's concentration is directly proportional to a solution's absorbance. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter. Beer Lambert Law Graph Explained.
From www.youtube.com
Derivation of Beer Lambert Law YouTube Beer Lambert Law Graph Explained beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law suggests that a plot of absorbance vs. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law states that a chemical's concentration is directly proportional to a solution's. Beer Lambert Law Graph Explained.
From sciencenotes.org
Beer's Law Equation and Example Beer Lambert Law Graph Explained “the path length and concentration of a chemical are directly proportional to its absorption of light.” One of the most basic of these. beer's law is an equation that relates light's attenuation to a material's properties. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. You may use this relation. Beer Lambert Law Graph Explained.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Graph Explained \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. You may use this. Beer Lambert Law Graph Explained.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation Beer Lambert Law Graph Explained in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. beer's law is an equation that relates light's attenuation to a material's properties. One of the most basic of these. In some cases. Beer Lambert Law Graph Explained.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Graph Explained since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. “the path length and concentration of a chemical are directly proportional to its absorption of light.” in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. One of the most basic of these.. Beer Lambert Law Graph Explained.
From exoxwjcgv.blob.core.windows.net
Beer's Law Graph Maker at Paul Bernstein blog Beer Lambert Law Graph Explained since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer's law is an equation that relates light's attenuation to a material's properties. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer lamberts law states a relationship between the. Beer Lambert Law Graph Explained.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer Lambert Law Graph Explained \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. beer’s law suggests that a plot of absorbance vs. “the path length and concentration of a chemical are directly. Beer Lambert Law Graph Explained.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer Lambert Law Graph Explained while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. beer's law is an equation that relates light's attenuation to a material's properties. beer lamberts law. Beer Lambert Law Graph Explained.
From byjus.com
State and explain Beer Lambert Law. Beer Lambert Law Graph Explained while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer’s law suggests that a plot of. Beer Lambert Law Graph Explained.
From www.researchgate.net
Schematics demonstrating the original BeerLambert Law and Modified Beer Lambert Law Graph Explained since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. “the path length and concentration of a chemical are directly proportional to its absorption of light.” \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. in his seminal 1852 paper, beer showed that. Beer Lambert Law Graph Explained.
From www.youtube.com
BeerLambert law in easy way YouTube Beer Lambert Law Graph Explained In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. beer's law is an equation that relates light's attenuation to a material's properties. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. The law states that. Beer Lambert Law Graph Explained.
From www.youtube.com
Introduction to UVVis Spectroscopy 03 BeerLambert Law YouTube Beer Lambert Law Graph Explained beer’s law suggests that a plot of absorbance vs. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly. Beer Lambert Law Graph Explained.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Beer Lambert Law Graph Explained beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer's law is an equation that relates light's attenuation to a material's properties. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. The law states that a chemical's. Beer Lambert Law Graph Explained.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer Lambert Law Graph Explained while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. beer’s law suggests that a plot of absorbance vs. One of the most basic of these. The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer lamberts law states a relationship between the attenuation. Beer Lambert Law Graph Explained.
From www.youtube.com
Beer Lambert Law simplest explanation animation YouTube Beer Lambert Law Graph Explained \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. beer's law is an equation that relates light's attenuation to a material's properties. beer’s law suggests that a plot of absorbance vs.. Beer Lambert Law Graph Explained.
From www.thoughtco.com
Beer's Law Definition and Equation Beer Lambert Law Graph Explained One of the most basic of these. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. since the concentration, path length and molar absorptivity are all. Beer Lambert Law Graph Explained.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer Lambert Law Graph Explained The law states that a chemical's concentration is directly proportional to a solution's absorbance. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a). Beer Lambert Law Graph Explained.
From testbook.com
Derivation of BeerLambert Law Statement, Formula, Graph, Uses Beer Lambert Law Graph Explained You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. since the concentration, path. Beer Lambert Law Graph Explained.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer Lambert Law Graph Explained \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law states that a chemical's concentration is directly proportional to a solution's absorbance. One of the most basic of these. beer lamberts law states a. Beer Lambert Law Graph Explained.
From chem.libretexts.org
10.2 Spectroscopy Based on Absorption Chemistry LibreTexts Beer Lambert Law Graph Explained In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law. Beer Lambert Law Graph Explained.
From www.youtube.com
Beer Lambert law derivation and usage YouTube Beer Lambert Law Graph Explained beer's law is an equation that relates light's attenuation to a material's properties. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The law states that a chemical's concentration is directly proportional to a solution's absorbance. since the concentration, path length and molar absorptivity are all directly proportional to the. Beer Lambert Law Graph Explained.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer Lambert Law Graph Explained beer's law is an equation that relates light's attenuation to a material's properties. in his seminal 1852 paper, beer showed that the transmittance of light through a cuvette is constant within. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation, graphs. One of the most basic of these. “the. Beer Lambert Law Graph Explained.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Graph Explained beer’s law suggests that a plot of absorbance vs. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer's law is an equation that relates light's attenuation to a material's properties. while studying this topic you will learn about beer’s law, lambert’s law, beer lambert’s law, its derivation,. Beer Lambert Law Graph Explained.
From www.youtube.com
Spectroscopy Beer Lambert's Law YouTube Beer Lambert Law Graph Explained beer’s law suggests that a plot of absorbance vs. In some cases a beer’s law plot deviates from this ideal behavior (see figure \(\pageindex{9}\)), and such deviations from linearity are divided into. You may use this relation to determine a chemical species' concentration in a solution using a colorimeter or spectrophotometer. One of the most basic of these. . Beer Lambert Law Graph Explained.
From thechemistrynotes.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer Lambert Law Graph Explained One of the most basic of these. “the path length and concentration of a chemical are directly proportional to its absorption of light.” The law states that a chemical's concentration is directly proportional to a solution's absorbance. beer's law is an equation that relates light's attenuation to a material's properties. In some cases a beer’s law plot deviates from. Beer Lambert Law Graph Explained.