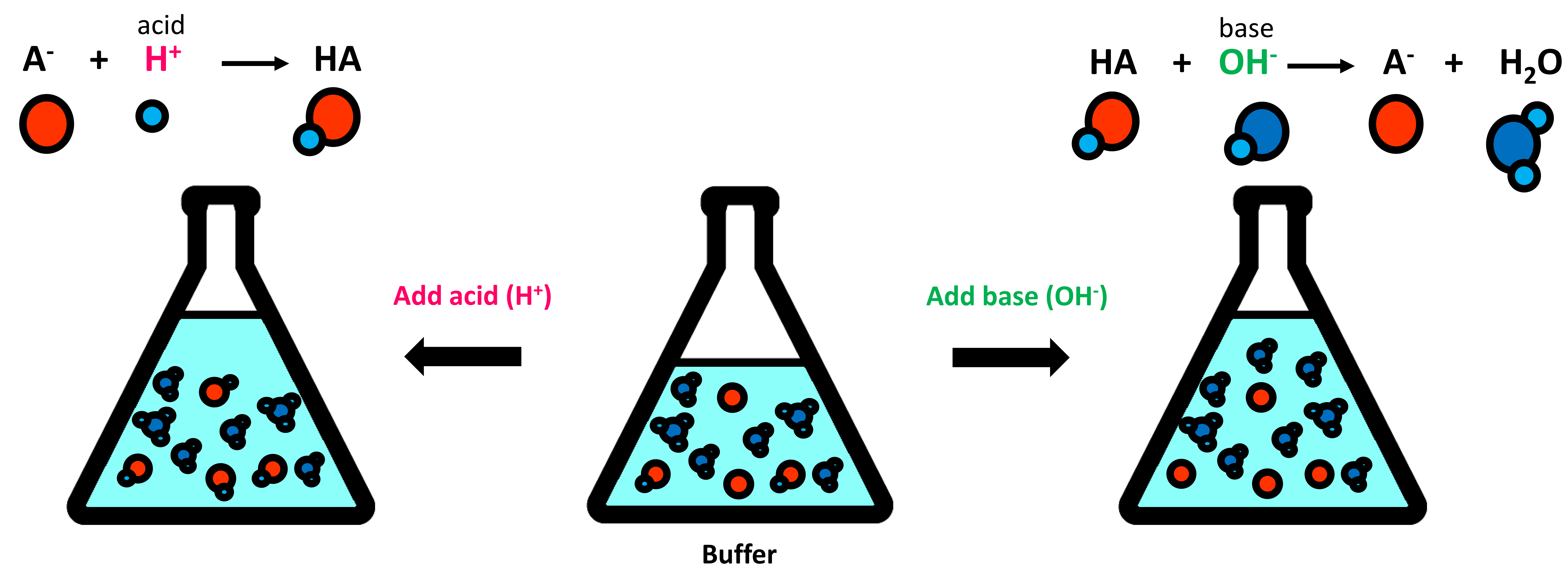
Buffer Vs Reagent . the use of buffers that mimic biological solutions is a foundation of biochemical and biophysical studies. Adding a strong electrolyte that. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. We provide six grades of buffers indicated. wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. Whether your application needs a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. what is a buffer solution? buffers are solutions that resist a change in ph after adding an acid or a base. buffers work well only for limited amounts of added strong acid or base. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.
from goldbio.com
buffers are solutions that resist a change in ph after adding an acid or a base. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffer grade indicates the quality and impurity levels appropriate for different uses. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. Adding a strong electrolyte that. while buffer concentrations do not always have to be exact, their ph and type must be correct, made. Whether your application needs a.
What is a Biological Buffer and How to Choose the Best Buffer for Your
Buffer Vs Reagent buffer grade indicates the quality and impurity levels appropriate for different uses. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. A buffer solution is one which resists changes in ph when small quantities of an acid or an. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. buffers work well only for limited amounts of added strong acid or base. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. buffer grade indicates the quality and impurity levels appropriate for different uses. wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. buffers are solutions that resist a change in ph after adding an acid or a base. we offer an extensive range of reagents and buffer solutions for your routine laboratory work. We provide six grades of buffers indicated. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. Whether your application needs a. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of.
From www.medicalexpo.com
Buffer solution reagent LXAB1 Moss Inc. for research Buffer Vs Reagent this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. Adding a strong electrolyte that. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. figure 12.2 “the actions of buffers.” buffers can react with both strong. Buffer Vs Reagent.
From www.youtube.com
What's the difference between a 'reactant' and a 'reagent'? YouTube Buffer Vs Reagent For example, 1 l of a. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffers work well only for limited amounts of added strong acid or base. a mixture. Buffer Vs Reagent.
From www.slideserve.com
PPT Titration and Buffers PowerPoint Presentation, free download ID Buffer Vs Reagent figure 12.2 “the actions of buffers.” buffers can react with both strong acids (top) and strong bases (side) to minimize large changes in ph. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. a mixture of a weak acid and its conjugate base (or a. Buffer Vs Reagent.
From www.differencebetween.com
Difference Between Catalyst and Reagent Compare the Difference Buffer Vs Reagent Adding a strong electrolyte that. buffer grade indicates the quality and impurity levels appropriate for different uses. For example, 1 l of a. buffers work well only for limited amounts of added strong acid or base. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffer capacity depends on the. Buffer Vs Reagent.
From www.biosharp.cn
RIPA Lysis Buffer Buffer Vs Reagent We provide six grades of buffers indicated. buffers are solutions that resist a change in ph after adding an acid or a base. the use of buffers that mimic biological solutions is a foundation of biochemical and biophysical studies. what is a buffer solution? buffer grade indicates the quality and impurity levels appropriate for different uses.. Buffer Vs Reagent.
From www.fishersci.com
Thermo Scientific Pierce IP Lysis BufferProtein Analysis Reagents Buffer Vs Reagent Whether your application needs a. we offer an extensive range of reagents and buffer solutions for your routine laboratory work. A buffer solution is one which resists changes in ph when small quantities of an acid or an. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent EB Omega Biotek, Inc. for research Buffer Vs Reagent A buffer solution is one which resists changes in ph when small quantities of an acid or an. figure 12.2 “the actions of buffers.” buffers can react with both strong acids (top) and strong bases (side) to minimize large changes in ph. buffer grade indicates the quality and impurity levels appropriate for different uses. buffer capacity depends. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent Sykam for biochemistry / liquid Buffer Vs Reagent buffer grade indicates the quality and impurity levels appropriate for different uses. we offer an extensive range of reagents and buffer solutions for your routine laboratory work. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. figure 12.2 “the actions of buffers.” buffers can react. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent SuperDissol™ Dakewe (Shenzhen) Medical Buffer Vs Reagent buffers are solutions that resist a change in ph after adding an acid or a base. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Once either solute is all reacted, the solution is no. Buffer Vs Reagent.
From www.welgene.com
20X SSC Buffer (ML01201) Welgene Buffer Vs Reagent while buffer concentrations do not always have to be exact, their ph and type must be correct, made. wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. buffer grade indicates. Buffer Vs Reagent.
From www.alamy.com
Buffer reagent hires stock photography and images Alamy Buffer Vs Reagent Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. Adding a strong electrolyte that. Whether your application needs a. A buffer solution is one which resists changes in ph when small quantities of an acid or an. buffers work well only for limited amounts of added strong acid or base. Buffers contain. Buffer Vs Reagent.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your Buffer Vs Reagent We provide six grades of buffers indicated. buffers work well only for limited amounts of added strong acid or base. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. buffers are solutions that resist a change in ph after adding an acid or a base. . Buffer Vs Reagent.
From pediaa.com
Difference Between Reactant and Reagent Definition, Properties, Examples Buffer Vs Reagent For example, 1 l of a. while buffer concentrations do not always have to be exact, their ph and type must be correct, made. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and. Buffer Vs Reagent.
From www.researchgate.net
Absorption spectra of (a) HMBATSC Vs Buffer blank (b) [V(V)HMBATSC] Vs Buffer Vs Reagent this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers work well only for limited amounts of added strong acid or. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent PC Zhejiang Xinke Medical Technology Buffer Vs Reagent what is a buffer solution? wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. buffer grade indicates the quality and impurity levels appropriate for different uses. buffers work well only for limited amounts of added strong acid or base. a mixture of a weak acid and its conjugate. Buffer Vs Reagent.
From webmis.highland.cc.il.us
Buffers Buffer Vs Reagent the use of buffers that mimic biological solutions is a foundation of biochemical and biophysical studies. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is. Buffer Vs Reagent.
From www.indiamart.com
Buffer Reagent at best price in New Delhi by Royal Chem ID 12653314930 Buffer Vs Reagent Adding a strong electrolyte that. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. a mixture of a weak acid and its conjugate base (or a. Buffer Vs Reagent.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Buffer Vs Reagent For example, 1 l of a. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. while buffer concentrations do not always have to be exact, their ph and type must be correct, made. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and. Buffer Vs Reagent.
From iwofr.org
¿Qué es un reactivo? Definición y ejemplos IWOFR Buffer Vs Reagent A buffer solution is one which resists changes in ph when small quantities of an acid or an. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). Whether your application needs a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent 18600 series Waters Ges.m.b.H for Buffer Vs Reagent a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic and basic. a mixture of a weak acid and its conjugate base. Buffer Vs Reagent.
From www.indiamart.com
280ml Thermo Scientific pH Buffer Solution, Grade Standard Reagent Buffer Vs Reagent Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). buffer grade indicates the quality and impurity levels appropriate for different uses. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. buffers are solutions that. Buffer Vs Reagent.
From ecpltd.com.au
Analytical Reagent Buffer Solution pH4 ECP Buffer Vs Reagent what is a buffer solution? Adding a strong electrolyte that. wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. We provide six grades of buffers indicated. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.. Buffer Vs Reagent.
From www.bio-world.com
MES Buffer 0.1M, pH 4.7 (4432319) bioWORLD Buffer Vs Reagent Whether your application needs a. Adding a strong electrolyte that. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. For example, 1 l of a. We provide six grades of buffers indicated. figure 12.2 “the actions of buffers.” buffers can react with both. Buffer Vs Reagent.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffer Vs Reagent wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. We provide six grades of buffers indicated. buffers are solutions that resist a change in ph after adding an acid or a base. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. For example, 1. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent PDR048 Omega Biotek, Inc. for research Buffer Vs Reagent a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. while buffer concentrations do not always have to be exact, their. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent JXK004 Zhejiang Xinke Medical Technology Buffer Vs Reagent buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. For example, 1 l of a. buffers work well only for limited amounts of added strong acid or base. Whether your application needs a. a mixture of a weak acid and its conjugate base (or a mixture. Buffer Vs Reagent.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Vs Reagent Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. while buffer concentrations do not always have to be exact, their ph and type must be correct, made. buffers are solutions that. Buffer Vs Reagent.
From www.chegg.com
Solved Consider the following questions about buffer Buffer Vs Reagent the use of buffers that mimic biological solutions is a foundation of biochemical and biophysical studies. buffers work well only for limited amounts of added strong acid or base. For example, 1 l of a. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is. Buffer Vs Reagent.
From www.slideserve.com
PPT DEFINITIONS OF ACIDS & BASES PowerPoint Presentation, free Buffer Vs Reagent Adding a strong electrolyte that. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes. buffer grade indicates the quality and impurity levels appropriate for different uses. we offer an extensive range of reagents and buffer solutions for your routine laboratory work. the use of buffers that mimic biological solutions is. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent kit sparQ QuantaBio for molecular biology Buffer Vs Reagent buffers are solutions that resist a change in ph after adding an acid or a base. buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer. what is a buffer solution? buffers work well only for limited amounts of added strong acid or base. wherever the. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent Zhejiang Xinke Medical Technology for Buffer Vs Reagent buffers work well only for limited amounts of added strong acid or base. we offer an extensive range of reagents and buffer solutions for your routine laboratory work. wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. A buffer solution is one which resists changes in ph when small quantities. Buffer Vs Reagent.
From thecontentauthority.com
Reactant vs Reagent Differences And Uses For Each One Buffer Vs Reagent while buffer concentrations do not always have to be exact, their ph and type must be correct, made. the use of buffers that mimic biological solutions is a foundation of biochemical and biophysical studies. buffers are solutions that resist a change in ph after adding an acid or a base. buffer grade indicates the quality and. Buffer Vs Reagent.
From www.medicalexpo.com
Buffer solution reagent 003821 YSI Inc. calibration / pH Buffer Vs Reagent A buffer solution is one which resists changes in ph when small quantities of an acid or an. buffers are solutions that resist a change in ph after adding an acid or a base. buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of. figure 12.2 “the. Buffer Vs Reagent.
From www.goldstarmedicals.com
Durable Buffer Solution Reagent Sale or Rent Near Me Goldstar Medical Buffer Vs Reagent buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture. Buffers contain a weak acid (\(ha\)) and its conjugate weak base (\(a^−\)). We provide six grades of buffers indicated. this chemistry tutorial introduces you to buffers by learning the definition of a buffer, and the components of acidic. Buffer Vs Reagent.
From www.bio-world.com
Sodium Chloride Peptone Solution Buffered with 1 Tween 80, Sterile Buffer Vs Reagent wherever the use of ammonium hydroxide or an acid is prescribed with no indication of strength. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. A buffer solution is one which resists changes in ph when small quantities of an acid or an.. Buffer Vs Reagent.