Glycidyl Methacrylate Carboxyl . epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. Containing both an epoxide and an acrylate groups,. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid):
from www.semanticscholar.org
in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. Containing both an epoxide and an acrylate groups,. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as.
Figure 4 from Reaction of glycidyl methacrylate at the hydroxyl and
Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): Containing both an epoxide and an acrylate groups,. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low.
From phasetransfercatalysis.com
Selective Esterification of Methacrylic Acid with Epichlorohydrin Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route. Glycidyl Methacrylate Carboxyl.
From pubs.rsc.org
Postpolymerization modification reactions of poly(glycidyl Glycidyl Methacrylate Carboxyl glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. glycidyl methacrylate (gma). Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 3 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. reaction. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
Scheme 2 Illustration of side reaction possible between the epoxy Glycidyl Methacrylate Carboxyl selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 9 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. glycidyl methacrylate (gma) is an ester of methacrylic acid. Glycidyl Methacrylate Carboxyl.
From pubs.acs.org
Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Groups Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. epoxide. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
(PDF) Clearing the mechanism of reaction of glycidyl methacrylate at Glycidyl Methacrylate Carboxyl we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. Containing both an. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 1 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. Hydrophilicity of hmcf was efficiently enhanced after. Glycidyl Methacrylate Carboxyl.
From alchetron.com
Glycidyl methacrylate Alchetron, The Free Social Encyclopedia Glycidyl Methacrylate Carboxyl poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. glycidyl methacrylate (gma) is. Glycidyl Methacrylate Carboxyl.
From pubs.rsc.org
Postpolymerization modification reactions of poly(glycidyl Glycidyl Methacrylate Carboxyl poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. Containing both an epoxide and an acrylate groups,. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. epoxide ring opening reaction is a route that explains. Glycidyl Methacrylate Carboxyl.
From www.polymersource.ca
Poly(glycidyl methacrylate) Glycidyl Methacrylate Carboxyl selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. poly (glycidyl methacrylate) (pgma) is prone to modifications. Glycidyl Methacrylate Carboxyl.
From www.freepng.fr
Glycidyl Méthacrylate, Lacide, Méthacrylate De Méthyle PNG Glycidyl Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. Hydrophilicity of hmcf. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
Scheme 1. HNC/ONC glycidyl methacrylate (GMA) grafting. Download Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide. Glycidyl Methacrylate Carboxyl.
From www.hybridplastics.com
HC0407.13 Glycidyl Methacryl POSS Hybrid Plastics Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. poly(glycidyl methacrylate) (pgma) is one. Glycidyl Methacrylate Carboxyl.
From www.fishersci.se
Glycidyl methacrylate, 97, stabilized, Thermo Scientific Chemicals Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. we prepared a matrix resin. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 1 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. poly(glycidyl methacrylate) (pgma) is one of the most interesting. Glycidyl Methacrylate Carboxyl.
From sielc.com
Glycidyl methacrylate SIELC Technologies Glycidyl Methacrylate Carboxyl we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. Containing both an epoxide and. Glycidyl Methacrylate Carboxyl.
From www.shandongbiotech.com
Glycidyl methacrylate Shandong Biotech Glycidyl Methacrylate Carboxyl Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side.. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
(PDF) Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Glycidyl Methacrylate Carboxyl Containing both an epoxide and an acrylate groups,. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. in this work, glycidyl methacrylate (gma) was polymerised and grafted. Glycidyl Methacrylate Carboxyl.
From pubs.rsc.org
Postpolymerization modification reactions of poly(glycidyl Glycidyl Methacrylate Carboxyl glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route that. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
Reaction sequences for the synthesis of PMASiNP decorated and Glycidyl Methacrylate Carboxyl glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide ring opening reaction is a route that explains. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. Containing both. Glycidyl Methacrylate Carboxyl.
From www.echemi.com
Buy Glycidyl Methacrylate 98 Pharma Grade 98 from Zhejiang Zetian Glycidyl Methacrylate Carboxyl poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. epoxide ring opening reaction is a route that explains the chemical modification of. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 4 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. Containing both an epoxide and an acrylate groups,. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
Chemical structures of bisphenol Aglycidyl methacrylate (bisGMA Glycidyl Methacrylate Carboxyl selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. . Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 1 from Monodisperse carboxylfunctionalized poly(ethylene glycol Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups. Glycidyl Methacrylate Carboxyl.
From www.researchgate.net
(PDF) Reaction of Glycidyl Methacrylate at the Hydroxyl and Carboxylic Glycidyl Methacrylate Carboxyl selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side.. Glycidyl Methacrylate Carboxyl.
From onlinelibrary.wiley.com
Monodisperse Carboxyl‐Functionalized Poly(Ethylene Glycol)‐Coated Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. in this work, glycidyl. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 5 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. Containing both an epoxide and an. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 13 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl poly (glycidyl methacrylate) (pgma) is prone to modifications with different functional groups, magnetic. poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. Containing both an epoxide and. Glycidyl Methacrylate Carboxyl.
From journal.polymer-korea.or.kr
Keywords photocurable resin, acrylmodified oligomer, crosslinking Glycidyl Methacrylate Carboxyl glycidyl methacrylate (gma) is a chemical compound that is synthesized and stored under specific conditions for use in. we prepared a matrix resin structured by udma, which is a high viscosity base monomer with imino groups, and by a low. Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. in this work, glycidyl methacrylate (gma). Glycidyl Methacrylate Carboxyl.
From vdocuments.mx
Modification of copolymers using nucleophilic reactions between Glycidyl Methacrylate Carboxyl Hydrophilicity of hmcf was efficiently enhanced after acid activation and grafting. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate.. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
Figure 3 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl poly(glycidyl methacrylate) (pgma) is one of the most interesting functional macromolecules with side. selective polymerization of glycidyl methacrylate (gma), which has two polymerizable functional groups such as. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of. Glycidyl Methacrylate Carboxyl.
From www.semanticscholar.org
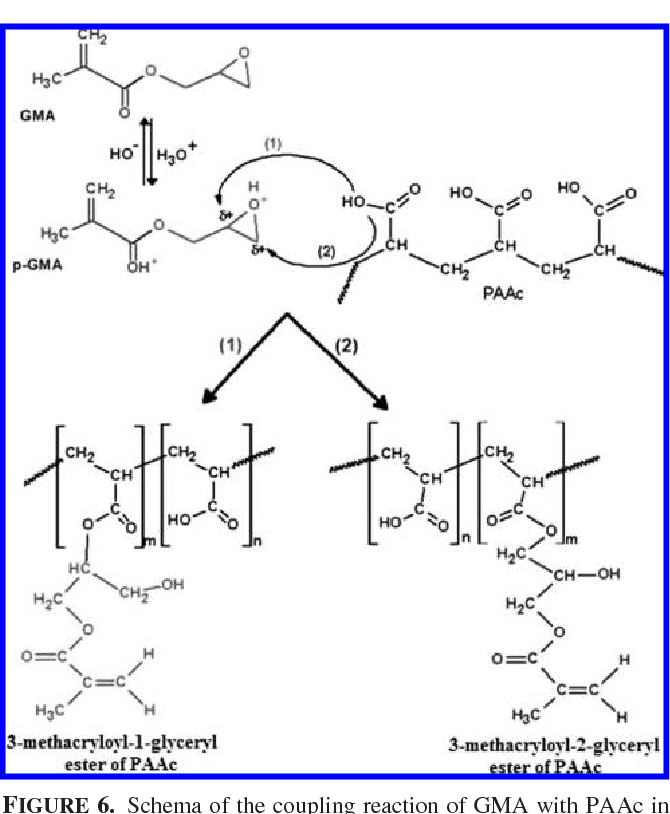
Figure 6 from Reaction of glycidyl methacrylate at the hydroxyl and Glycidyl Methacrylate Carboxyl glycidyl methacrylate (gma) is an ester of methacrylic acid and glycidol. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate (gma) polymers. epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. Containing both an epoxide and an acrylate groups,. glycidyl methacrylate (gma) is a. Glycidyl Methacrylate Carboxyl.
From www.mdpi.com
Polymers Free FullText Development of BisphenolAGlycidyl Glycidyl Methacrylate Carboxyl epoxide ring opening reaction is a route that explains the chemical modification of glycidyl methacrylate. reaction of glycidyl methacrylate at the hydroxyl and carboxylic groups of poly(vinyl alcohol) and poly(acrylic acid): in this work, glycidyl methacrylate (gma) was polymerised and grafted onto the surface of carbon fiber. Containing both an epoxide and an acrylate groups,. poly(glycidyl. Glycidyl Methacrylate Carboxyl.