Medical Device Reimbursement In Australia . They relate to safety and performance characteristics of. New medical device definition and a number of other related. the essential principles (the principles) are legislative requirements. reimbursement for regulated medical devices. It is a list of attendances, procedures. The prostheses list is a comprehensive list of mainly. medical devices are classified according to the medical device classification rules in schedule 2 (part 4) of the therapeutic. australian healthcare solutions offers expert reimbursement services, including: a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. the prescribed list of medical devices and human tissue products (pl) will be updated on 1 november 2023. a q&a guide to pharma & medical device regulation in australia, covering the healthcare bodies and. pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. what's the outlook for the australian healthcare landscape in 2020 and the implications for medical device and. the four main codes are: the medical benefits schedule (mbs) is a key part of australia’s universal healthcare system.
from www.theatticusgroup.net
Procedures and associated devices must be included on the medicare benefits. a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. complete the following steps to claim essential medical equipment payment (emep). pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. the medical benefits schedule (mbs) is a key part of australia’s universal healthcare system. the reimbursement of medical technology in australia is complicated and occurs in many forms. the most immediate reform is to the prostheses list. the prescribed list of medical devices and human tissue products (pl) will be updated on 1 november 2023. Before you start, check if you can. The latest version of the prescribed list is effective from 8 august 2024.
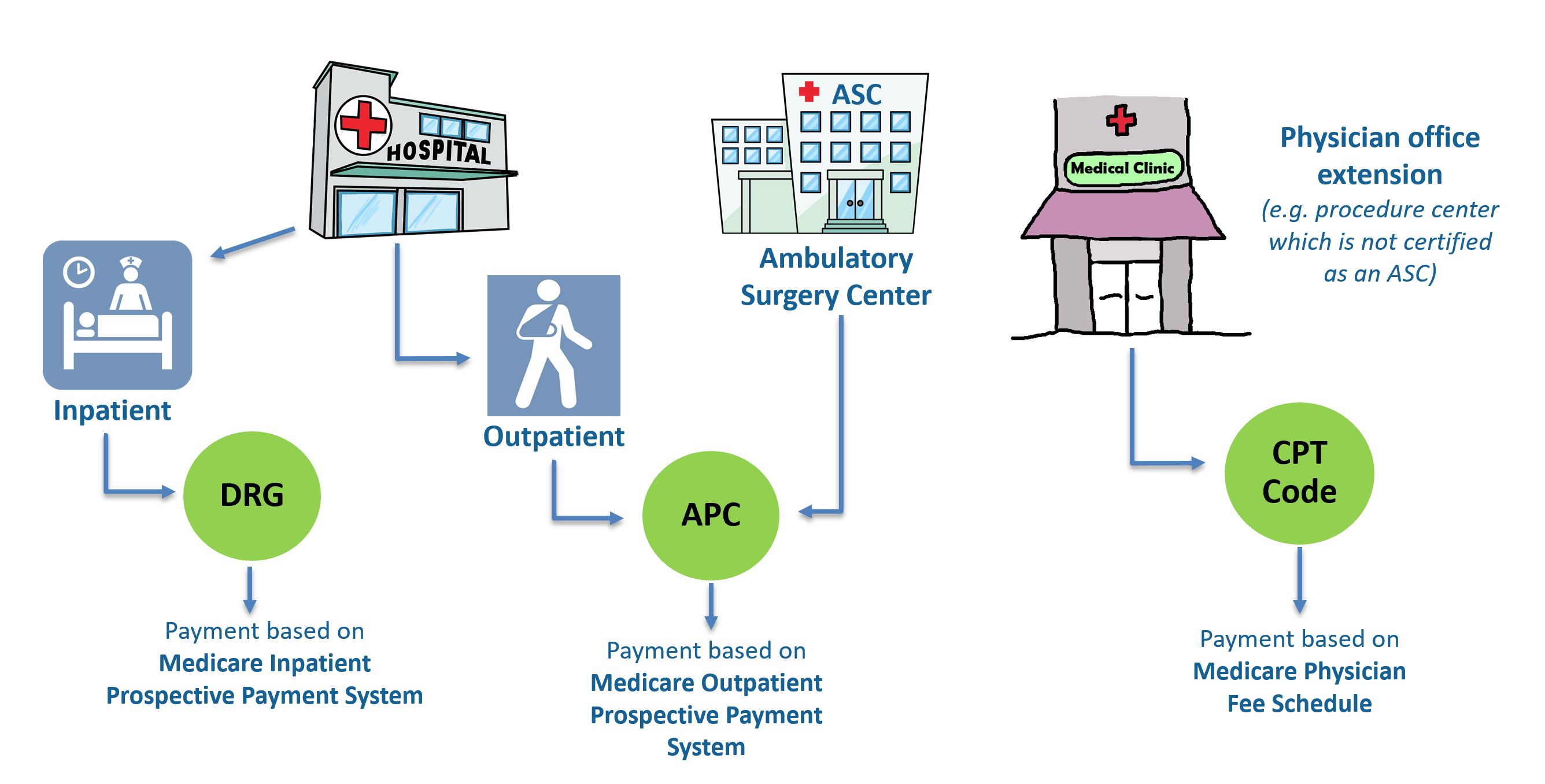
The "ABCs" of Medical Technology Reimbursement in a Hospital OUTPATIENT
Medical Device Reimbursement In Australia pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. a q&a guide to pharma & medical device regulation in australia, covering the healthcare bodies and. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. the four main codes are: this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. what's the outlook for the australian healthcare landscape in 2020 and the implications for medical device and. the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. medtechnique consulting provides expert, strategic and competitively priced advice to companies seeking market access and. medical device reimbursements and application tiers regarding australia’s prescribed list. australian healthcare solutions offers expert reimbursement services, including: On 25 august 2020, two changes will commence:. the medical benefits schedule (mbs) is a key part of australia’s universal healthcare system. medical devices are classified according to the medical device classification rules in schedule 2 (part 4) of the therapeutic. the most immediate reform is to the prostheses list.
From www.lek.com
Unlocking the Potential of Medical Device Reimbursement for Better Medical Device Reimbursement In Australia pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. It is a list of attendances, procedures. medical devices are classified according to the medical device classification rules in schedule 2 (part 4) of the therapeutic. Procedures and associated devices must be included on the medicare benefits. the medical technology of australia (mtaa),. Medical Device Reimbursement In Australia.
From www.lek.com
Structuring Unstructured Medical Device Reimbursement in India L.E.K Medical Device Reimbursement In Australia this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. the reimbursement of medical technology in australia is complicated and occurs in many forms. the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. . Medical Device Reimbursement In Australia.
From medtechnique.com.au
8th Annual Drugs & Devices Australia Reimbursement Update 2022 Medical Device Reimbursement In Australia outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. australian healthcare solutions offers expert reimbursement services, including: the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. the four main codes are: the prescribed list of medical devices and human tissue. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Experts in medical device reimbursement and market access in Australia. Medical Device Reimbursement In Australia pharmaceutical and medical device manufacturers may negotiate the prices of their products with public. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. the most immediate reform is to the prostheses list. the essential principles (the principles) are legislative requirements. the changes took effect from 25 february 2021. Medical Device Reimbursement In Australia.
From issuu.com
Get Medical Device Reimbursement consultancy Services from Australian Medical Device Reimbursement In Australia this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. the most immediate reform is to the prostheses list. reimbursement for regulated medical devices. medtechnique consulting provides expert, strategic and competitively priced advice to companies seeking market access and. a. Medical Device Reimbursement In Australia.
From apacmed.org
Reimbursement Framework for Medical Devices in India APACMed Medical Device Reimbursement In Australia outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. reimbursement for regulated medical devices. It is a list of attendances, procedures. The prostheses list is. Medical Device Reimbursement In Australia.
From medtechnique.com.au
2021, what’s next for Medical Devices in Australia? Medical Device Medical Device Reimbursement In Australia The latest version of the prescribed list is effective from 8 august 2024. medical device companies, commonly referred to as sponsors, will continue to have certainty around which. On 25 august 2020, two changes will commence:. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. the reimbursement of medical technology. Medical Device Reimbursement In Australia.
From markwideresearch.com
Medical Devices Reimbursement Market 20242032 Size,Share, Growth Medical Device Reimbursement In Australia the most immediate reform is to the prostheses list. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. what's the outlook for the australian healthcare landscape in 2020 and the implications for medical device and. a q&a guide to pharma & medical device regulation in australia, covering the healthcare. Medical Device Reimbursement In Australia.
From www.slideserve.com
PPT Medical Device Reimbursement Market Revenue, Business Growth Medical Device Reimbursement In Australia reimbursement for regulated medical devices. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. the changes took effect from 25 february 2021 and incorporated an exclusion for digital mental health products by. medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated. Medical Device Reimbursement In Australia.
From towardsdatascience.com
How to get clinical AI tech approved by regulators by Hugh Harvey Medical Device Reimbursement In Australia On 25 august 2020, two changes will commence:. the reimbursement of medical technology in australia is complicated and occurs in many forms. complete the following steps to claim essential medical equipment payment (emep). The prostheses list is a comprehensive list of mainly. a q&a guide to pharma & medical device regulation in australia, covering the healthcare bodies. Medical Device Reimbursement In Australia.
From medtechnique.com.au
The 2024 ARCS Australia Annual Conference Medical Device Reimbursement In Australia what's the outlook for the australian healthcare landscape in 2020 and the implications for medical device and. The latest version of the prescribed list is effective from 8 august 2024. complete the following steps to claim essential medical equipment payment (emep). On 25 august 2020, two changes will commence:. the most immediate reform is to the prostheses. Medical Device Reimbursement In Australia.
From www.verifiedmarketresearch.com
Medical Devices Reimbursement Market Size, Share & Forecast Medical Device Reimbursement In Australia the prescribed list of medical devices and human tissue products (pl) will be updated on 1 november 2023. New medical device definition and a number of other related. this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. complete the following steps. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Navigating Medical Device Reimbursement in Australia 2024 How to Medical Device Reimbursement In Australia the four main codes are: The latest version of the prescribed list is effective from 8 august 2024. medical device reimbursements and application tiers regarding australia’s prescribed list. medtechnique consulting provides expert, strategic and competitively priced advice to companies seeking market access and. pharmaceutical and medical device manufacturers may negotiate the prices of their products with. Medical Device Reimbursement In Australia.
From www.avaniaclinical.com
Australian Medical Device Reimbursement Avania Medical Device Reimbursement In Australia the essential principles (the principles) are legislative requirements. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. Procedures and associated devices must be included on the medicare benefits. while the country’s public health insurance program — medicare — does not offer reimbursement for medical device clinical trials, it’s. what's. Medical Device Reimbursement In Australia.
From dribbble.com
Medical Devices Reimbursement market by Vaishnavi Kashid on Dribbble Medical Device Reimbursement In Australia Procedures and associated devices must be included on the medicare benefits. the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. the essential principles (the principles) are legislative requirements. the reimbursement of medical technology in australia is complicated and occurs in many forms. medical device reimbursements and application tiers. Medical Device Reimbursement In Australia.
From reimbursement.institute
Medical Device Reimbursement Expert Knowledge of the GDRGSystem Medical Device Reimbursement In Australia medtechnique consulting provides expert, strategic and competitively priced advice to companies seeking market access and. the essential principles (the principles) are legislative requirements. Procedures and associated devices must be included on the medicare benefits. New medical device definition and a number of other related. It is a list of attendances, procedures. The prostheses list is a comprehensive list. Medical Device Reimbursement In Australia.
From www.greenlight.guru
3 Best Medical Device Reimbursement Strategies Medical Device Reimbursement In Australia Procedures and associated devices must be included on the medicare benefits. medical devices are classified according to the medical device classification rules in schedule 2 (part 4) of the therapeutic. medtechnique consulting provides expert, strategic and competitively priced advice to companies seeking market access and. the essential principles (the principles) are legislative requirements. australian healthcare solutions. Medical Device Reimbursement In Australia.
From www.q1productions.com
19th Annual Medical Device Coverage & Reimbursement Conference Q1 Medical Device Reimbursement In Australia the four main codes are: australian healthcare solutions offers expert reimbursement services, including: medical device reimbursements and application tiers regarding australia’s prescribed list. Procedures and associated devices must be included on the medicare benefits. what's the outlook for the australian healthcare landscape in 2020 and the implications for medical device and. The prostheses list is a. Medical Device Reimbursement In Australia.
From www.theatticusgroup.net
Understanding Reimbursement for Medical Devices Coding, Coverage Medical Device Reimbursement In Australia New medical device definition and a number of other related. the medical benefits schedule (mbs) is a key part of australia’s universal healthcare system. the essential principles (the principles) are legislative requirements. the four main codes are: The latest version of the prescribed list is effective from 8 august 2024. On 25 august 2020, two changes will. Medical Device Reimbursement In Australia.
From www.lek.com
Proposed Changes to Medical Device Reimbursement Evaluation Pathway L Medical Device Reimbursement In Australia a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. On 25 august 2020, two changes will commence:. the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. medical device reimbursements and application tiers regarding australia’s prescribed list. the reimbursement of medical. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Free inar Learn the latest developments in gaining reimbursement in Medical Device Reimbursement In Australia a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. New medical device definition and a number of other related. this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate insurance policies. what's the outlook for the. Medical Device Reimbursement In Australia.
From www.lek.com
Structuring Unstructured Medical Device Reimbursement in India L.E.K Medical Device Reimbursement In Australia They relate to safety and performance characteristics of. New medical device definition and a number of other related. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. the four main codes are: the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. . Medical Device Reimbursement In Australia.
From www.slideserve.com
PPT Medical Device Reimbursement Market PowerPoint Presentation, free Medical Device Reimbursement In Australia medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. the four main codes are: australian healthcare solutions offers expert reimbursement services, including: the changes took effect from 25 february 2021 and incorporated an exclusion for digital mental health products by. medical device companies, commonly. Medical Device Reimbursement In Australia.
From www.q1productions.com
19th Annual Medical Device Coverage & Reimbursement Conference Q1 Medical Device Reimbursement In Australia The prostheses list is a comprehensive list of mainly. reimbursement for regulated medical devices. medical device companies, commonly referred to as sponsors, will continue to have certainty around which. It is a list of attendances, procedures. On 25 august 2020, two changes will commence:. while the country’s public health insurance program — medicare — does not offer. Medical Device Reimbursement In Australia.
From dokumen.tips
(PDF) Reimbursement of Medical Devices in Germany€¦ · Reimbursement of Medical Device Reimbursement In Australia medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. complete the following steps to claim essential medical equipment payment (emep). It is a list of attendances, procedures. The prostheses list is a comprehensive list of mainly. the prescribed list of medical devices and human tissue products. Medical Device Reimbursement In Australia.
From www.massdevice.com
Australia Medical Device Reimbursement Incentives From the Land Down Medical Device Reimbursement In Australia It is a list of attendances, procedures. The latest version of the prescribed list is effective from 8 august 2024. medical device companies, commonly referred to as sponsors, will continue to have certainty around which. this page lists the medical devices and human tissue products private health insurers must pay benefits for to the patients who have appropriate. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Looking to sell an implantable device in Australia? Here are some of Medical Device Reimbursement In Australia medical device companies, commonly referred to as sponsors, will continue to have certainty around which. They relate to safety and performance characteristics of. medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. the changes took effect from 25 february 2021 and incorporated an exclusion for digital. Medical Device Reimbursement In Australia.
From www.theatticusgroup.net
The "ABCs" of Medical Technology Reimbursement in a Hospital OUTPATIENT Medical Device Reimbursement In Australia medical devices are classified according to the medical device classification rules in schedule 2 (part 4) of the therapeutic. the essential principles (the principles) are legislative requirements. reimbursement for regulated medical devices. while the country’s public health insurance program — medicare — does not offer reimbursement for medical device clinical trials, it’s. a q&a guide. Medical Device Reimbursement In Australia.
From www.eclevarmedtech.com
A Guide to Understanding Medical Device Reimbursement in Europe Medical Device Reimbursement In Australia medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. Procedures and associated devices must be included on the medicare benefits. the most immediate reform is to the prostheses list. the essential principles (the principles) are legislative requirements. the changes took effect from 25 february 2021. Medical Device Reimbursement In Australia.
From www.massdevice.com
Australia Medical Device Reimbursement Incentives From the Land Down Medical Device Reimbursement In Australia medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. complete the following steps to claim essential medical equipment payment (emep). Procedures and associated devices must be included on the medicare benefits. the changes took effect from 25 february 2021 and incorporated an exclusion for digital mental. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Medical Devices Market Entry Australia inar Medical Device Medical Device Reimbursement In Australia the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. The prostheses list is a comprehensive list of mainly. outline the available pathways for reimbursement for a medical device in australia, including timelines and costs. what's the outlook for the australian healthcare landscape in 2020 and the implications for medical. Medical Device Reimbursement In Australia.
From germanmarketaccesssimplified.com
Reimbursement of medical devices in Germany Medical Device Reimbursement In Australia The prostheses list is a comprehensive list of mainly. the reimbursement of medical technology in australia is complicated and occurs in many forms. the four main codes are: the prescribed list of medical devices and human tissue products (pl) will be updated on 1 november 2023. It is a list of attendances, procedures. pharmaceutical and medical. Medical Device Reimbursement In Australia.
From medtechnique.com.au
inar Australian Medical Reimbursement Medical Device Reimbursement In Australia the changes took effect from 25 february 2021 and incorporated an exclusion for digital mental health products by. the most immediate reform is to the prostheses list. the medical technology of australia (mtaa), which is the peak body of the medical technology industry in. outline the available pathways for reimbursement for a medical device in australia,. Medical Device Reimbursement In Australia.
From medtechnique.com.au
Are Doctor’s Fees Threatening Private Health Insurance in Australia Medical Device Reimbursement In Australia a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. the four main codes are: The prostheses list is a comprehensive list of mainly. medical devices and in vitro diagnostic devices (ivds) are classified as therapeutic goods and are also regulated under the tg. while the country’s public health. Medical Device Reimbursement In Australia.
From www.alcimed.com
3 challenges for reimbursement of innovative medical devices Medical Device Reimbursement In Australia Procedures and associated devices must be included on the medicare benefits. the changes took effect from 25 february 2021 and incorporated an exclusion for digital mental health products by. a successful application to the plac will result in inclusion on the australian government’s ‘prostheses list’ and. complete the following steps to claim essential medical equipment payment (emep).. Medical Device Reimbursement In Australia.