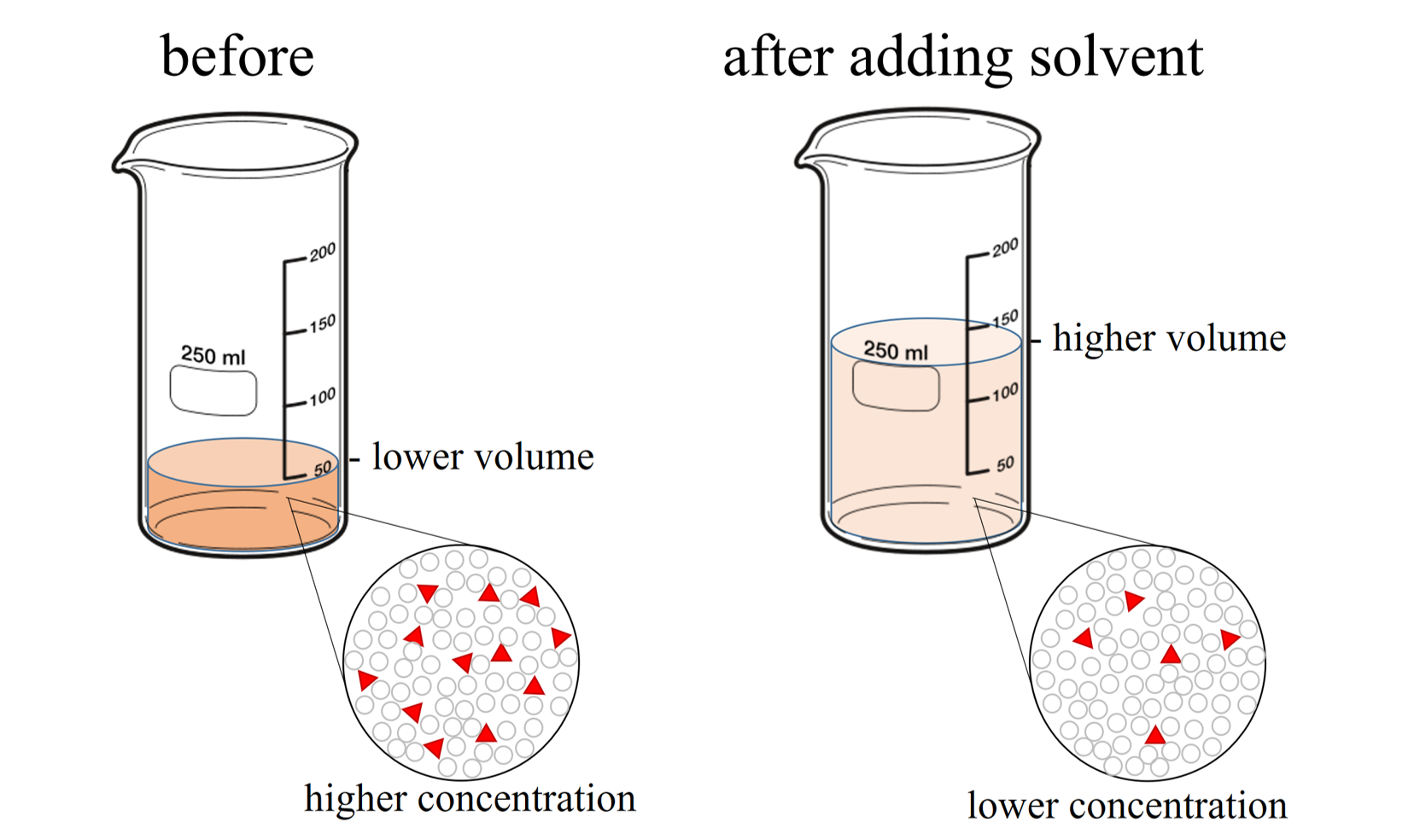
Titration And Dilution Practice Problems . 1) if it takes 54 ml of 0.1 m. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. dilution problems, chemistry, molarity & concentration examples, formula & equations Problem \(\pageindex{2}\) what does it mean when. this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. Chemists have many methods for determining the quantity of a substance. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. if you're seeing this message, it means we're having trouble loading external resources on our website. Find the requested quantities in the following problems: you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. General chemistry ii jasperse buffers/titrations/solubility. If it takes 54 ml of 0.1 m naoh. If you're behind a web filter,. Find the requested quantities in the following problems: in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using.
from chem.libretexts.org
this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. the concentration and the volumes change in a dilution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Chemists have many methods for determining the quantity of a substance. You may be given beginning or ending. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Intro to general chemistry 3h 48m. dilution practice problems & example problems. A flask contains 10.0 ml of an unknown concentration of h2so4. Problem \(\pageindex{2}\) what does it mean when.
14.7 Solution Dilution Chemistry LibreTexts
Titration And Dilution Practice Problems you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. Use our revision notes to understand titration calculations for your a level chemistry course using worked. Problem \(\pageindex{2}\) what does it mean when. this site will produce an unlimited number of practice problems for calulating dilutions. General chemistry ii jasperse buffers/titrations/solubility. A flask contains 10.0 ml of an unknown concentration of h2so4. By the end of this section, you will be able to: If it takes 54 ml of 0.1 m naoh. if you're seeing this message, it means we're having trouble loading external resources on our website. If volumes are additive and 95.0 ml of 0.55. this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. calculate or sketch titration curves for the following (unbalanced) redox titration reactions at 25 o c. Chemists have many methods for determining the quantity of a substance. Find the requested quantities in the following problems: dilution practice problems & example problems.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Titration And Dilution Practice Problems the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Chemists have many methods for determining the quantity of a substance. this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. Find the requested quantities in the following problems: By. Titration And Dilution Practice Problems.
From www.studocu.com
TitrationandDilution I. INTRODUCTION Neutralization of acid and Titration And Dilution Practice Problems 1) if it takes 54 ml of 0.1 m. If you're behind a web filter,. A flask contains 10.0 ml of an unknown concentration of h2so4. General chemistry ii jasperse buffers/titrations/solubility. the concentration and the volumes change in a dilution. Intro to general chemistry 3h 48m. Find the requested quantities in the following problems: If volumes are additive and. Titration And Dilution Practice Problems.
From learningisidro.z13.web.core.windows.net
Making Dilutions Worksheets Titration And Dilution Practice Problems this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. If it takes 54 ml of 0.1 m naoh. dilution problems, chemistry, molarity & concentration examples, formula & equations Use our revision notes to understand titration calculations for your a level chemistry course using worked. Problem \(\pageindex{2}\) what does. Titration And Dilution Practice Problems.
From dxosnjafu.blob.core.windows.net
Dilution Calculation Videos at Iva Fugate blog Titration And Dilution Practice Problems 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Find the requested quantities in the following problems: 1) if it takes 54 ml of 0.1 m. dilution problems, chemistry, molarity & concentration examples, formula & equations the example below demonstrates the technique to solve a titration problem for. Titration And Dilution Practice Problems.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Titration And Dilution Practice Problems If volumes are additive and 95.0 ml of 0.55. dilution problems, chemistry, molarity & concentration examples, formula & equations this site will produce an unlimited number of practice problems for calulating dilutions. Intro to general chemistry 3h 48m. 1) if it takes 54 ml of 0.1 m. you should dilute the 133 ml of an 7.90 m. Titration And Dilution Practice Problems.
From www.chegg.com
Solved AcidBase Titration Practice Problems 1) Calculate Titration And Dilution Practice Problems Problem \(\pageindex{2}\) what does it mean when. Use our revision notes to understand titration calculations for your a level chemistry course using worked. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. Chemists have many methods for determining the quantity of a substance. the example below demonstrates the technique. Titration And Dilution Practice Problems.
From lessonmagicchampart.z14.web.core.windows.net
Titration Math Practice Problems Titration And Dilution Practice Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. If volumes are additive and 95.0 ml of 0.55. you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. Chemists have many methods for determining. Titration And Dilution Practice Problems.
From exopodmtv.blob.core.windows.net
Saline Dilution Test For Acanthocytes at Gloria Fearon blog Titration And Dilution Practice Problems dilution problems, chemistry, molarity & concentration examples, formula & equations If it takes 54 ml of 0.1 m naoh. this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. . Titration And Dilution Practice Problems.
From slideplayer.com
Chapter 8 Solutions 8.5 Molarity and Dilution. ppt download Titration And Dilution Practice Problems Use our revision notes to understand titration calculations for your a level chemistry course using worked. 1) if it takes 54 ml of 0.1 m. If you're behind a web filter,. If it takes 54 ml of 0.1 m naoh. Find the requested quantities in the following problems: the concentration and the volumes change in a dilution. dilution. Titration And Dilution Practice Problems.
From www.chemistrylearner.com
Free Printable Acids and Bases Titration Worksheets Titration And Dilution Practice Problems Problem \(\pageindex{2}\) what does it mean when. By the end of this section, you will be able to: A flask contains 10.0 ml of an unknown concentration of h2so4. this site will produce an unlimited number of practice problems for calulating dilutions. If volumes are additive and 95.0 ml of 0.55. Use our revision notes to understand titration calculations. Titration And Dilution Practice Problems.
From www.chemistryspace.com
Molarity, Dilutions, and Titration • Practice Problems Titration And Dilution Practice Problems If you're behind a web filter,. Chemists have many methods for determining the quantity of a substance. if you're seeing this message, it means we're having trouble loading external resources on our website. Find the requested quantities in the following problems: If volumes are additive and 95.0 ml of 0.55. the example below demonstrates the technique to solve. Titration And Dilution Practice Problems.
From www.studocu.com
Titration Curve Practice Problems MATH + SCIENCE INITIATIVE Titration Titration And Dilution Practice Problems Find the requested quantities in the following problems: Use our revision notes to understand titration calculations for your a level chemistry course using worked. calculate or sketch titration curves for the following (unbalanced) redox titration reactions at 25 o c. Chemists have many methods for determining the quantity of a substance. the example below demonstrates the technique to. Titration And Dilution Practice Problems.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co Titration And Dilution Practice Problems By the end of this section, you will be able to: Find the requested quantities in the following problems: you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. 1) if it takes 54 ml of 0.1 m. Find the requested quantities in the following problems: If it takes 54 ml of 0.1. Titration And Dilution Practice Problems.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Titration And Dilution Practice Problems dilution problems, chemistry, molarity & concentration examples, formula & equations 1) if it takes 54 ml of 0.1 m. Find the requested quantities in the following problems: the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. You may be given beginning or ending. Chemists have many methods for. Titration And Dilution Practice Problems.
From quizzlistreplevies.z13.web.core.windows.net
Molarity And Dilution Worksheet Titration And Dilution Practice Problems General chemistry ii jasperse buffers/titrations/solubility. dilution problems, chemistry, molarity & concentration examples, formula & equations 1) if it takes 54 ml of 0.1 m. If it takes 54 ml of 0.1 m naoh. the concentration and the volumes change in a dilution. this online quiz is intended to give you extra practice in calculating analyte concentrations and. Titration And Dilution Practice Problems.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Titration And Dilution Practice Problems Intro to general chemistry 3h 48m. calculate or sketch titration curves for the following (unbalanced) redox titration reactions at 25 o c. Find the requested quantities in the following problems: Use our revision notes to understand titration calculations for your a level chemistry course using worked. Chemists have many methods for determining the quantity of a substance. Find the. Titration And Dilution Practice Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration And Dilution Practice Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. If you're behind a web filter,. Find the requested quantities in the following problems: 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl. Titration And Dilution Practice Problems.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Titration And Dilution Practice Problems if you're seeing this message, it means we're having trouble loading external resources on our website. the concentration and the volumes change in a dilution. dilution practice problems & example problems. Find the requested quantities in the following problems: If volumes are additive and 95.0 ml of 0.55. Use our revision notes to understand titration calculations for. Titration And Dilution Practice Problems.
From www.studocu.com
Dissolution Aqueous solutions, dilution, titration, practice problems Titration And Dilution Practice Problems this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. dilution problems, chemistry, molarity & concentration examples, formula & equations in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. By. Titration And Dilution Practice Problems.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Titration And Dilution Practice Problems dilution practice problems & example problems. General chemistry ii jasperse buffers/titrations/solubility. If volumes are additive and 95.0 ml of 0.55. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. 1) if it takes 54 ml of 0.1 m. You may be given beginning or ending. in this. Titration And Dilution Practice Problems.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration And Dilution Practice Problems If it takes 54 ml of 0.1 m naoh. Chemists have many methods for determining the quantity of a substance. the concentration and the volumes change in a dilution. Intro to general chemistry 3h 48m. Find the requested quantities in the following problems: If you're behind a web filter,. you should dilute the 133 ml of an 7.90. Titration And Dilution Practice Problems.
From materialmagicvantassel.z21.web.core.windows.net
Acid Base Calculator Chemistry Titration And Dilution Practice Problems dilution problems, chemistry, molarity & concentration examples, formula & equations Problem \(\pageindex{2}\) what does it mean when. dilution practice problems & example problems. Use our revision notes to understand titration calculations for your a level chemistry course using worked. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. Titration And Dilution Practice Problems.
From printablehaferbrotwp.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration And Dilution Practice Problems this site will produce an unlimited number of practice problems for calulating dilutions. A flask contains 10.0 ml of an unknown concentration of h2so4. you should dilute the 133 ml of an 7.90 m cucl 2 solution to 1620 ml. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. Titration And Dilution Practice Problems.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration And Dilution Practice Problems the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. If it takes 54 ml of 0.1 m naoh. you should dilute the 133 ml of an 7.90 m cucl. Titration And Dilution Practice Problems.
From exogoqrkx.blob.core.windows.net
Titration Class 12Th Practical at Dolores Parker blog Titration And Dilution Practice Problems If volumes are additive and 95.0 ml of 0.55. Intro to general chemistry 3h 48m. Chemists have many methods for determining the quantity of a substance. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. If you're behind a web filter,. this site will produce an unlimited number. Titration And Dilution Practice Problems.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Titration And Dilution Practice Problems If you're behind a web filter,. this online quiz is intended to give you extra practice in calculating analyte concentrations and titrant volumes for a. 1) if it takes 54 ml of 0.1 m. calculate or sketch titration curves for the following (unbalanced) redox titration reactions at 25 o c. By the end of this section, you will. Titration And Dilution Practice Problems.
From printablelibfinance.z13.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration And Dilution Practice Problems the concentration and the volumes change in a dilution. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Chemists have many. Titration And Dilution Practice Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration And Dilution Practice Problems Find the requested quantities in the following problems: A flask contains 10.0 ml of an unknown concentration of h2so4. dilution practice problems & example problems. the concentration and the volumes change in a dilution. calculate or sketch titration curves for the following (unbalanced) redox titration reactions at 25 o c. General chemistry ii jasperse buffers/titrations/solubility. Use our. Titration And Dilution Practice Problems.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration And Dilution Practice Problems the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. If it takes 54 ml of 0.1 m naoh. 1) if it takes 54 ml of 0.1 m. if you're seeing this message, it means we're having trouble loading external resources on our website. dilution practice problems &. Titration And Dilution Practice Problems.
From learningzonelisannin.z14.web.core.windows.net
Chemistry Titration Questions And Answers Titration And Dilution Practice Problems Use our revision notes to understand titration calculations for your a level chemistry course using worked. Intro to general chemistry 3h 48m. the concentration and the volumes change in a dilution. Find the requested quantities in the following problems: dilution practice problems & example problems. in this section, we will see how to perform calculations to predict. Titration And Dilution Practice Problems.
From www.chegg.com
Titration Results Of 1/10 Dilution. Titration And Dilution Practice Problems Use our revision notes to understand titration calculations for your a level chemistry course using worked. the example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. Intro to general chemistry 3h 48m. Find the requested quantities in the following problems: 1) it takes 83 ml of a 0.45 m naoh. Titration And Dilution Practice Problems.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Practical Questions And Answers Pdf Titration And Dilution Practice Problems this site will produce an unlimited number of practice problems for calulating dilutions. 1) it takes 83 ml of a 0.45 m naoh solution to neutralize 235 ml of an hcl solution. By the end of this section, you will be able to: If it takes 54 ml of 0.1 m naoh. if you're seeing this message, it. Titration And Dilution Practice Problems.
From sciencesavers.info
System, Calculator, Methodology, Makes use of, Examples sciencesavers Titration And Dilution Practice Problems if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter,. Find the requested quantities in the following problems: 1) if it takes 54 ml of 0.1 m. in this section, we will see how to perform calculations to predict the ph at any point in a. Titration And Dilution Practice Problems.
From dxooyumci.blob.core.windows.net
Titration Practice Problems Chemistry at Gloria Ogorman blog Titration And Dilution Practice Problems A flask contains 10.0 ml of an unknown concentration of h2so4. Intro to general chemistry 3h 48m. the concentration and the volumes change in a dilution. You may be given beginning or ending. Use our revision notes to understand titration calculations for your a level chemistry course using worked. this online quiz is intended to give you extra. Titration And Dilution Practice Problems.
From www.studocu.com
Acid Base TitrationsSet3Key Acid/Base Titration Practice Problems Titration And Dilution Practice Problems If volumes are additive and 95.0 ml of 0.55. You may be given beginning or ending. Problem \(\pageindex{2}\) what does it mean when. If you're behind a web filter,. A flask contains 10.0 ml of an unknown concentration of h2so4. if you're seeing this message, it means we're having trouble loading external resources on our website. Intro to general. Titration And Dilution Practice Problems.