Zinc Iodide Formation . zinc iodide is a colorless solid. In this article, learn more about the. zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. the iodide formation reaction mechanism. the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal pyrazolylbis(thioimidazolyl)borate ligand. the chemical formula of the anhydrous form of zinc iodide is zni2 z n i 2. It is a reducing agent. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. zinc iodide is a iodide of zinc. The masses of iodide and zinc reacted will be measured along with. The in situ and ex situ data allow deriving a detailed reaction. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. This means that each molecule of zinc.
from www.nagwa.com
It is a reducing agent. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? here, the zinc atom forms an ionic bond with two iodine atoms, which is reflected in the subscript ‘2’. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. The masses of iodide and zinc reacted will be measured along with. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. the chemical formula of the anhydrous form of zinc iodide is zni2 z n i 2. zinc iodide is a colorless solid. the iodide formation reaction mechanism. as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction.
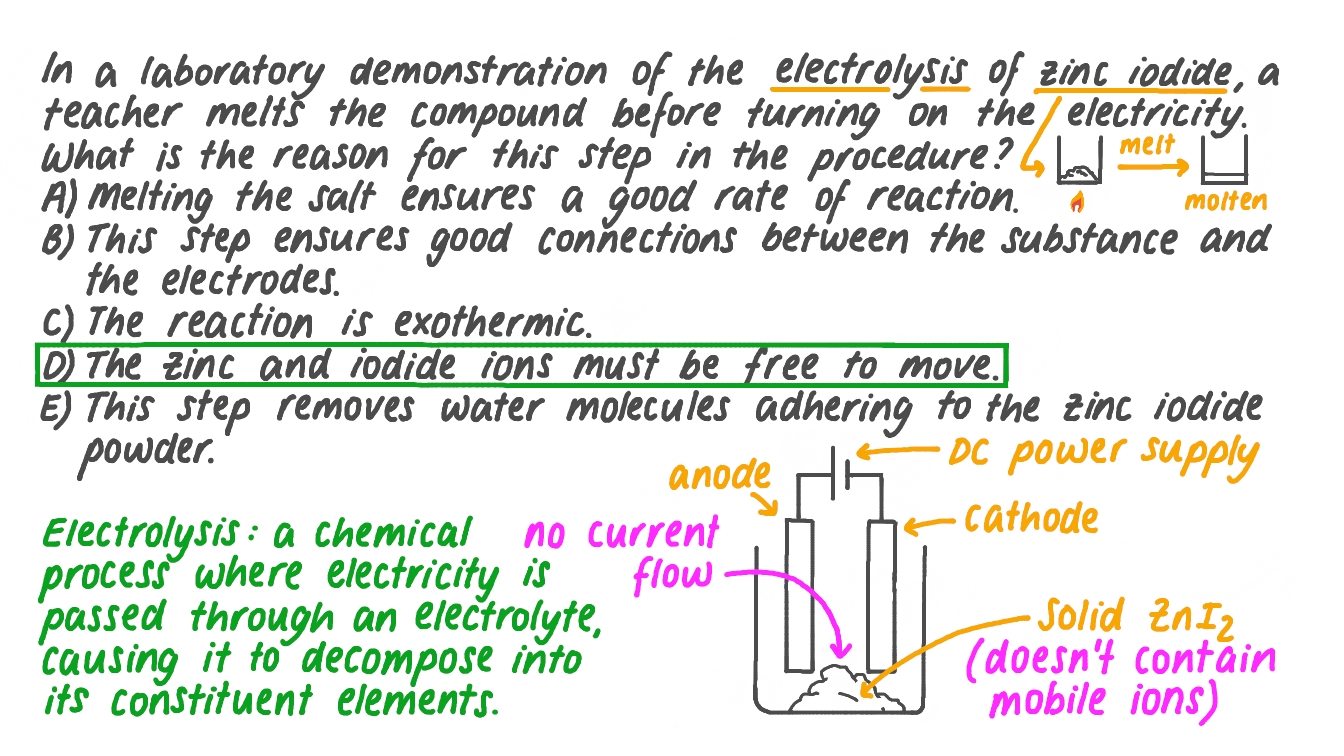
Question Video Identifying the Reason for Melting Zinc Iodide before
Zinc Iodide Formation In this article, learn more about the. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. In this article, learn more about the. It reacts with bases to make zinc hydroxide. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. The masses of iodide and zinc reacted will be measured along with. The in situ and ex situ data allow deriving a detailed reaction. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. zinc iodide is a colorless solid. the iodide formation reaction mechanism. Zn + i 2 → zni 2. this gradual addition of the plant extract facilitated the formation of undoped zno nanoparticles through a. in this experiment, elemental zinc will be reacted with elemental iodine to produce zinc iodide. This means that each molecule of zinc. it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. It is a reducing agent.
From www.alamy.com
Chemical structure of zinc iodide hires stock photography and images Zinc Iodide Formation zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. It is a reducing agent. This means that each molecule of zinc. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. Zinc and iodine, when mixed with distilled water in a test tube, will react. Zinc Iodide Formation.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc Iodide Formation cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal pyrazolylbis(thioimidazolyl)borate ligand. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators. Zinc Iodide Formation.
From askfilo.com
In a reaction between zinc and iodine, zinc iodide is formed as the produ.. Zinc Iodide Formation cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. the iodide formation reaction mechanism. Zn²⁺ + 2i⁻ → zni2 what is. Zinc Iodide Formation.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, White Powder. Chemical Compound of Zinc and Zinc Iodide Formation The masses of iodide and zinc reacted will be measured along with. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. The experiment can be extended to show the decomposition of a compound into its elements. It is a reducing agent. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula. Zinc Iodide Formation.
From www.indiamart.com
Zinc Iodide at Rs 3014/kg Zinc Iodide in Mumbai ID 7994270648 Zinc Iodide Formation It reacts with bases to make zinc hydroxide. here, the zinc atom forms an ionic bond with two iodine atoms, which is reflected in the subscript ‘2’. zinc iodide is a colorless solid. The experiment can be extended to show the decomposition of a compound into its elements. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites. Zinc Iodide Formation.
From www.youtube.com
Zinc Iodide YouTube Zinc Iodide Formation This means that each molecule of zinc. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. cf3soo̶. Zinc Iodide Formation.
From pubs.rsc.org
Zinc iodide a mild and efficient catalyst for onepot synthesis of Zinc Iodide Formation It reacts with bases to make zinc hydroxide. The experiment can be extended to show the decomposition of a compound into its elements. zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. This. Zinc Iodide Formation.
From www.youtube.com
Formation of zinc iodide YouTube Zinc Iodide Formation the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal pyrazolylbis(thioimidazolyl)borate ligand. this gradual addition of the plant extract facilitated the formation of undoped zno nanoparticles through a. Zn + i 2 → zni 2. thermodynamic studies on zinc iodide and mercurous iodide. The experiment can be extended to show the decomposition. Zinc Iodide Formation.
From www.docsity.com
Synthesis and of Zinc Iodide in General Chemistry I CH Zinc Iodide Formation It reacts with bases to make zinc hydroxide. zinc iodide is a iodide of zinc. in this experiment, elemental zinc will be reacted with elemental iodine to produce zinc iodide. It is a reducing agent. The masses of iodide and zinc reacted will be measured along with. The in situ and ex situ data allow deriving a detailed. Zinc Iodide Formation.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses Zinc Iodide Formation The masses of iodide and zinc reacted will be measured along with. Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. It reacts with bases to make zinc hydroxide. discrete. Zinc Iodide Formation.
From dxoqfympp.blob.core.windows.net
Zinc Oxide Ionic Compound at Victoria Cann blog Zinc Iodide Formation the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. The in situ and ex situ data allow deriving a detailed reaction. zinc iodide is. Zinc Iodide Formation.
From exotwicqs.blob.core.windows.net
Iodine And Zinc Equation at Nancy Moore blog Zinc Iodide Formation this gradual addition of the plant extract facilitated the formation of undoped zno nanoparticles through a. Zn²⁺ + 2i⁻ → zni2 what is zni2 called? the formation of zinc iodide from its constituent ions can be represented by the chemical equation: Zinc is a metallic element with the atomic number 30. Zn + i 2 → zni 2.. Zinc Iodide Formation.
From studylib.net
Zinc Iodide Lab Zinc Iodide Formation Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. the chemical formula of the anhydrous form of zinc iodide is zni2 z n i 2. this gradual addition of the plant extract facilitated the. Zinc Iodide Formation.
From dxohphgdt.blob.core.windows.net
Copper Oxide Equation at Dora Longstreet blog Zinc Iodide Formation as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal pyrazolylbis(thioimidazolyl)borate ligand. it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. Zinc. Zinc Iodide Formation.
From anastasia-has-harrison.blogspot.com
Aluminum Iodide and Silver I Nitrate Net Ionic Equation Anastasiahas Zinc Iodide Formation The experiment can be extended to show the decomposition of a compound into its elements. This means that each molecule of zinc. zinc iodide is a colorless solid. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. zinc iodide is a iodide of zinc. the iodide. Zinc Iodide Formation.
From studylib.net
SYNTHESIS OF ZINC IODIDE Experiment 4 R. S. Nord Stephen DeMeo, Zinc Iodide Formation it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. Zn + i 2 → zni 2. It is a reducing agent. In this article, learn more about the. The masses of iodide and zinc reacted will be measured along with. thermodynamic studies on zinc iodide and. Zinc Iodide Formation.
From www.nagwa.com
Question Video Identifying the Reason for Melting Zinc Iodide before Zinc Iodide Formation an exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. zinc iodide is a colorless solid. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of. Zinc Iodide Formation.
From synthonix.com
Synthonix, Inc > Grignards and Zincs > 1350356523 (Tetrahydropyran Zinc Iodide Formation Zn + i 2 → zni 2. here, the zinc atom forms an ionic bond with two iodine atoms, which is reflected in the subscript ‘2’. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. Zni2 is called zinc iodide. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and. Zinc Iodide Formation.
From www.sdcychem.com
Zinc iodide CAS 10139476 SDCY CHEM Zinc Iodide Formation as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. zinc iodide is a iodide of zinc. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. Zinc is a metallic element with. Zinc Iodide Formation.
From www.grainger.com
10139476, F.W. 319.22, Zinc Iodide, Purified 39H157Z1073500GM10 Zinc Iodide Formation the chemical formula of the anhydrous form of zinc iodide is zni2 z n i 2. Zni2 is called zinc iodide. thermodynamic studies on zinc iodide and mercurous iodide. the formation of zinc iodide from its constituent ions can be represented by the chemical equation: zinc iodide is a iodide of zinc. The masses of iodide. Zinc Iodide Formation.
From cymitquimica.com
Zinc(II) Iodide CymitQuimica Zinc Iodide Formation zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. The in situ and ex situ data allow deriving a detailed reaction. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal. Zinc Iodide Formation.
From www.youtube.com
Finding the Empirical Formula For Zinc Iodide General Chemistry Zinc Iodide Formation the formation of zinc iodide from its constituent ions can be represented by the chemical equation: It is a reducing agent. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. in this experiment, elemental zinc will be reacted with elemental iodine to produce zinc iodide. In this article, learn more about the.. Zinc Iodide Formation.
From www.samaterials.com
Osmium Zinc Iodide (CAS 39386259) Zinc Iodide Formation Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. here, the zinc atom forms an ionic bond with two iodine atoms, which is reflected in the subscript ‘2’. zinc iodide is a iodide of zinc. The masses of iodide and zinc reacted will be measured along with. discrete mononuclear trispyrazolylborate. Zinc Iodide Formation.
From www.researchgate.net
(PDF) Synthesis and of Zinc Iodide Model Reactions for Zinc Iodide Formation it was found that zinc combined gold and silver as nanocomposite in “all” exhibited a superior anticancer activity over the other. zinc iodide is a iodide of zinc. Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. in this experiment, elemental zinc will be reacted with elemental iodine to produce. Zinc Iodide Formation.
From www.fishersci.se
Zinc iodide, 98, Thermo Scientific Chemicals Fisher Scientific Zinc Iodide Formation The in situ and ex situ data allow deriving a detailed reaction. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. here, the zinc atom forms an ionic bond with two iodine atoms, which is reflected in the subscript ‘2’. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by. Zinc Iodide Formation.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, White Powder. Stock Photo Image of heap Zinc Iodide Formation Zinc is a metallic element with the atomic number 30. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. zinc iodide is a iodide of zinc. cf3soo̶ vigorously suppresses molecular iodine. Zinc Iodide Formation.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, White Powder. Chemical Compound of Zinc and Zinc Iodide Formation The in situ and ex situ data allow deriving a detailed reaction. the catalytic site of ladh has been modeled using the mixed donor ns 2 tripodal pyrazolylbis(thioimidazolyl)borate ligand. the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and. in this experiment, elemental zinc will be reacted with elemental iodine to. Zinc Iodide Formation.
From www.researchgate.net
(PDF) Synthesis of Zinc Iodide Revisited Zinc Iodide Formation the formation of zinc iodide from its constituent ions can be represented by the chemical equation: It is a reducing agent. thermodynamic studies on zinc iodide and mercurous iodide. The masses of iodide and zinc reacted will be measured along with. this gradual addition of the plant extract facilitated the formation of undoped zno nanoparticles through a.. Zinc Iodide Formation.
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Zinc Iodide Formation This means that each molecule of zinc. Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. Zni2 is called zinc iodide. In this article, learn more about the. in this experiment, elemental zinc will be reacted with elemental iodine to produce zinc iodide. here, the zinc atom forms an ionic bond. Zinc Iodide Formation.
From www.exportersindia.com
Zinc Iodide, for Industrial at Best Price in Mumbai ID 6584201 Zinc Iodide Formation discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. zinc iodide is a colorless solid. The experiment can be extended to show the decomposition of a compound into its elements. It reacts with bases to make zinc. Zinc Iodide Formation.
From www.coursehero.com
. cadmium sulfide zinc iodide iron ( ) oxide lead Zinc Iodide Formation zinc iodide is a colorless solid. It is a reducing agent. the chemical formula of the anhydrous form of zinc iodide is zni2 z n i 2. as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. this gradual addition of the plant extract facilitated the formation of. Zinc Iodide Formation.
From edu.rsc.org
Exothermic redox reaction of zinc with iodine Experiment RSC Education Zinc Iodide Formation Zinc is a metallic element with the atomic number 30. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. as confirmed by a series of in situ and ex situ spectroscopy techniques, due to the favorable reaction. The in situ and ex situ data allow deriving a detailed. Zinc Iodide Formation.
From www.grainger.com
SPECTRUM Zinc Iodide, Purified 10139476, F.W. 319.22, ZnI2, Amber Zinc Iodide Formation zinc iodide is a colorless solid. thermodynamic studies on zinc iodide and mercurous iodide. Zinc and iodine, when mixed with distilled water in a test tube, will react spontaneously and. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. the iodide formation reaction mechanism. The in situ and ex situ data allow deriving a. Zinc Iodide Formation.
From www.indiamart.com
Powder Zinc Iodide, For Laboratory, 25 KG at Rs 5500/kg in Mumbai ID Zinc Iodide Formation zinc iodide is a iodide of zinc. cf3soo̶ vigorously suppresses molecular iodine formation in the perovskites by reducing it to iodide. This means that each molecule of zinc. zinc iodide is a chemical compound made of zinc and iodine with a molecular formula zni2. the iodide formation reaction mechanism. this gradual addition of the plant. Zinc Iodide Formation.
From www.axiomchemind.com
Iodide Zinc Iodide Anhydrous Manufacturer from Vadodara Zinc Iodide Formation zinc iodide is manufactured by the reaction of zinc and iodine while refluxing ether. the iodide formation reaction mechanism. the purpose of this article is to discuss two colorful reactions not widely used by chemical educators in high. discrete mononuclear trispyrazolylborate zinc cyanide complexes have been synthesized and structurally. Zn²⁺ + 2i⁻ → zni2 what is. Zinc Iodide Formation.