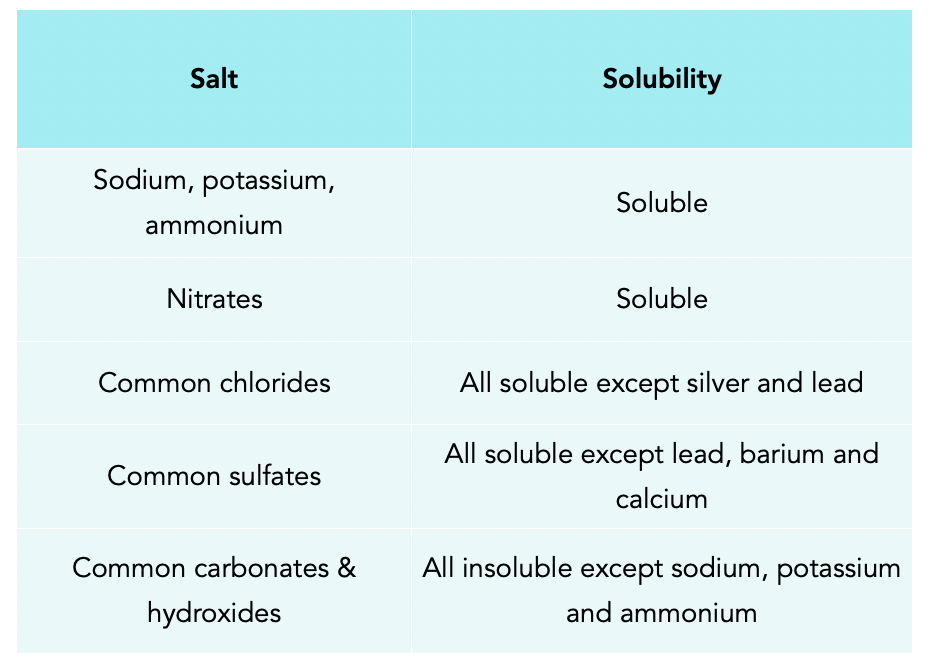
What Compounds Are Not Soluble In Water . When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Hydrocarbons are not very soluble in. But, solubility is not an. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Not all ionic compounds are soluble in water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Solubility rules can be used to predict whether a large number of ionic. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: For example, ethanol is soluble in water. However, to predict whether the compound will dissolve or not, we need to know a few general. A soluble chemical freely dissolves in a solvent in any ratio. In insoluble chemical does not dissolve in the solvent.
from ar.inspiredpencil.com
If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Solubility rules can be used to predict whether a large number of ionic. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. But, solubility is not an. However, to predict whether the compound will dissolve or not, we need to know a few general. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. A soluble chemical freely dissolves in a solvent in any ratio. Hydrocarbons are not very soluble in. For example, ethanol is soluble in water. Our article on solubility explains how ionic compounds dissolve in water to form a solution.
Solubility Chart Chemistry 11
What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Hydrocarbons are not very soluble in. Solubility rules can be used to predict whether a large number of ionic. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: However, to predict whether the compound will dissolve or not, we need to know a few general. A soluble chemical freely dissolves in a solvent in any ratio. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. In insoluble chemical does not dissolve in the solvent. But, solubility is not an. Our article on solubility explains how ionic compounds dissolve in water to form a solution. For example, ethanol is soluble in water. Not all ionic compounds are soluble in water.
From www.expii.com
Soluble vs. Insoluble — Comparison & Examples Expii What Compounds Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. For example, ethanol is soluble in water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. A soluble chemical freely dissolves in a solvent in any ratio. Not all ionic compounds are soluble in water. When some. What Compounds Are Not Soluble In Water.
From www.chegg.com
Solved Arrange the compounds from most soluble in water to What Compounds Are Not Soluble In Water In insoluble chemical does not dissolve in the solvent. Solubility rules can be used to predict whether a large number of ionic. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. A soluble chemical freely dissolves in a solvent in any ratio. For example, ethanol is soluble in. What Compounds Are Not Soluble In Water.
From www.chegg.com
Solved Arrange the organic compounds from most soluble in What Compounds Are Not Soluble In Water For example, ethanol is soluble in water. However, to predict whether the compound will dissolve or not, we need to know a few general. A soluble chemical freely dissolves in a solvent in any ratio. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. If we look at the structure. What Compounds Are Not Soluble In Water.
From www.coursehero.com
[Solved] . Which of the following compounds is soluble in water at room... Course Hero What Compounds Are Not Soluble In Water A soluble chemical freely dissolves in a solvent in any ratio. Our article on solubility explains how ionic compounds dissolve in water to form a solution. For example, ethanol is soluble in water. But, solubility is not an. However, to predict whether the compound will dissolve or not, we need to know a few general. Review solubility rules for common. What Compounds Are Not Soluble In Water.
From www.vrogue.co
Solved Activity 2 1 Solubility Chart Use The Chart Be vrogue.co What Compounds Are Not Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are soluble in water. But, solubility is not an. Solubility rules can be used to predict whether a large number of ionic. Our article on solubility explains how ionic compounds dissolve in water to form a solution.. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab PowerPoint Presentation ID1821173 What Compounds Are Not Soluble In Water Hydrocarbons are not very soluble in. In insoluble chemical does not dissolve in the solvent. Not all ionic compounds are soluble in water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields. What Compounds Are Not Soluble In Water.
From www.chegg.com
2. The molecule below is not soluble in water. [4 What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. For example, ethanol is soluble in water. But, solubility is not an. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Solubility rules can be used to predict whether. What Compounds Are Not Soluble In Water.
From www.pinterest.com
Solubility Rules Chart for Chemistry Classroom 11th chemistry, Chemistry classroom, Solubility What Compounds Are Not Soluble In Water For example, ethanol is soluble in water. Solubility rules can be used to predict whether a large number of ionic. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: However, to predict whether the compound will dissolve or not, we need to know a few general. In insoluble chemical does. What Compounds Are Not Soluble In Water.
From rayb78.github.io
Solubility In Water Chart What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. However, to predict whether the compound will dissolve or not, we need to know a few general. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Solubility rules can be used to predict. What Compounds Are Not Soluble In Water.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which Chloride Compound Is What Compounds Are Not Soluble In Water A soluble chemical freely dissolves in a solvent in any ratio. In insoluble chemical does not dissolve in the solvent. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: But, solubility. What Compounds Are Not Soluble In Water.
From www.chemistryspace.com
Solubility Rules for Ionic Compounds What Compounds Are Not Soluble In Water A soluble chemical freely dissolves in a solvent in any ratio. Hydrocarbons are not very soluble in. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are. What Compounds Are Not Soluble In Water.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps What Compounds Are Not Soluble In Water Hydrocarbons are not very soluble in. A soluble chemical freely dissolves in a solvent in any ratio. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. But, solubility is not an. In insoluble chemical does not dissolve in the solvent. However, to predict whether the compound will dissolve or not,. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility Rules and Precipitation Reactions PowerPoint Presentation ID9519440 What Compounds Are Not Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are soluble in water. A soluble chemical freely dissolves in a solvent in any ratio. Hydrocarbons are not very soluble in. When some substances are dissolved in water, they undergo either a physical or a chemical change. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Hydrocarbons are not very soluble in. A soluble chemical freely dissolves in a solvent in any ratio. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. For example, ethanol is soluble. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility Rules & Net Ionic Equations PowerPoint Presentation ID2740106 What Compounds Are Not Soluble In Water Hydrocarbons are not very soluble in. For example, ethanol is soluble in water. A soluble chemical freely dissolves in a solvent in any ratio. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: But, solubility is not an. Review solubility rules for common ionic compounds in water, including calcium carbonate,. What Compounds Are Not Soluble In Water.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic Compounds AII sodium (Na What Compounds Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. For example, ethanol is soluble in water. Not all ionic compounds are soluble in water. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Hydrocarbons are not very soluble in. A soluble chemical freely dissolves in a. What Compounds Are Not Soluble In Water.
From socratic.org
Substance A will not dissolve in water. What can be said about substance A? Socratic What Compounds Are Not Soluble In Water But, solubility is not an. Solubility rules can be used to predict whether a large number of ionic. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. In insoluble chemical does not dissolve in the solvent. If we look at the structure of compounds that dissolve in water, we can. What Compounds Are Not Soluble In Water.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: In insoluble chemical does not dissolve in the solvent. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that. What Compounds Are Not Soluble In Water.
From brainly.com
Which of the following molecules is least soluble in water? What Compounds Are Not Soluble In Water Hydrocarbons are not very soluble in. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are soluble in water. Solubility rules can be used to predict whether a large number of ionic. If we look at the structure of compounds that dissolve in water, we can. What Compounds Are Not Soluble In Water.
From www.numerade.com
SOLVED Which one of the following two compounds imaged below is expected to be more soluble in What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Hydrocarbons are not very soluble in. Not all ionic compounds are soluble in water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. A soluble chemical freely dissolves in. What Compounds Are Not Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Solubility rules can be used to predict whether a large number of ionic. Not all ionic compounds are soluble in water. Hydrocarbons are not very soluble in. In insoluble chemical does not dissolve in the solvent. However, to predict. What Compounds Are Not Soluble In Water.
From ar.inspiredpencil.com
Solubility Chart Chemistry 11 What Compounds Are Not Soluble In Water However, to predict whether the compound will dissolve or not, we need to know a few general. A soluble chemical freely dissolves in a solvent in any ratio. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Hydrocarbons are not very soluble in. Review solubility rules for common ionic compounds. What Compounds Are Not Soluble In Water.
From www.chegg.com
Solved Explain why this compound is not soluble in water. What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Not all ionic compounds are soluble in water. In insoluble chemical does not dissolve in the solvent. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Hydrocarbons are not very soluble. What Compounds Are Not Soluble In Water.
From www.chegg.com
Solved Which compounds are not soluble in water at room What Compounds Are Not Soluble In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. But, solubility is not an.. What Compounds Are Not Soluble In Water.
From bceweb.org
Solubility Of Compounds In Water Chart A Visual Reference of Charts Chart Master What Compounds Are Not Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. However,. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free download ID1820946 What Compounds Are Not Soluble In Water Solubility rules can be used to predict whether a large number of ionic. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. A soluble chemical freely dissolves in a solvent in any ratio. Our article on solubility explains how ionic compounds dissolve in water to form a solution.. What Compounds Are Not Soluble In Water.
From brainly.com
The compound below is expected to be soluble in water. True or False True False What Compounds Are Not Soluble In Water Hydrocarbons are not very soluble in. Solubility rules can be used to predict whether a large number of ionic. A soluble chemical freely dissolves in a solvent in any ratio. Not all ionic compounds are soluble in water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When some substances. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID1999793 What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: For example, ethanol is soluble in water. But, solubility is not an. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When some substances are dissolved in water, they undergo either. What Compounds Are Not Soluble In Water.
From casegrocooley.blogspot.com
Determine Whether Each Compound Is Soluble or Insoluble in Water. CasegroCooley What Compounds Are Not Soluble In Water In insoluble chemical does not dissolve in the solvent. A soluble chemical freely dissolves in a solvent in any ratio. Solubility rules can be used to predict whether a large number of ionic. Not all ionic compounds are soluble in water. But, solubility is not an. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate,. What Compounds Are Not Soluble In Water.
From www.slideserve.com
PPT A biological compound that is not soluble in water, but is soluble in nonpolar substances What Compounds Are Not Soluble In Water But, solubility is not an. A soluble chemical freely dissolves in a solvent in any ratio. For example, ethanol is soluble in water. Hydrocarbons are not very soluble in. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Not all ionic compounds are soluble in water. Our article on solubility. What Compounds Are Not Soluble In Water.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Solubility rules can be used to predict whether a large number of ionic. Not all ionic compounds are soluble in water. Hydrocarbons are not very soluble in. Our article on solubility explains how ionic compounds dissolve in water to form a. What Compounds Are Not Soluble In Water.
From oneclass.com
OneClass Rank the following organic compounds from most soluble in water to least soluble in What Compounds Are Not Soluble In Water If we look at the structure of compounds that dissolve in water, we can begin to see some trends: Hydrocarbons are not very soluble in. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Solubility rules can be used to predict whether a large number of ionic. When some substances are dissolved in water,. What Compounds Are Not Soluble In Water.
From www.chegg.com
Solved Which of the following compounds is not soluble in What Compounds Are Not Soluble In Water Our article on solubility explains how ionic compounds dissolve in water to form a solution. But, solubility is not an. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. However, to predict whether the compound will dissolve or not, we need to know a few general. Solubility rules. What Compounds Are Not Soluble In Water.
From www.numerade.com
SOLVED Arrange the organic compounds from most soluble in water to least soluble in water Most What Compounds Are Not Soluble In Water Our article on solubility explains how ionic compounds dissolve in water to form a solution. If we look at the structure of compounds that dissolve in water, we can begin to see some trends: When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. For example, ethanol is soluble. What Compounds Are Not Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Compounds Are Not Soluble In Water However, to predict whether the compound will dissolve or not, we need to know a few general. In insoluble chemical does not dissolve in the solvent. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions. What Compounds Are Not Soluble In Water.